Outcomes of non-hospitalized patients with COVID-19 versus seasonal influenza during the fall-winter 2022–2023 period
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-10833-6, Mar 2025
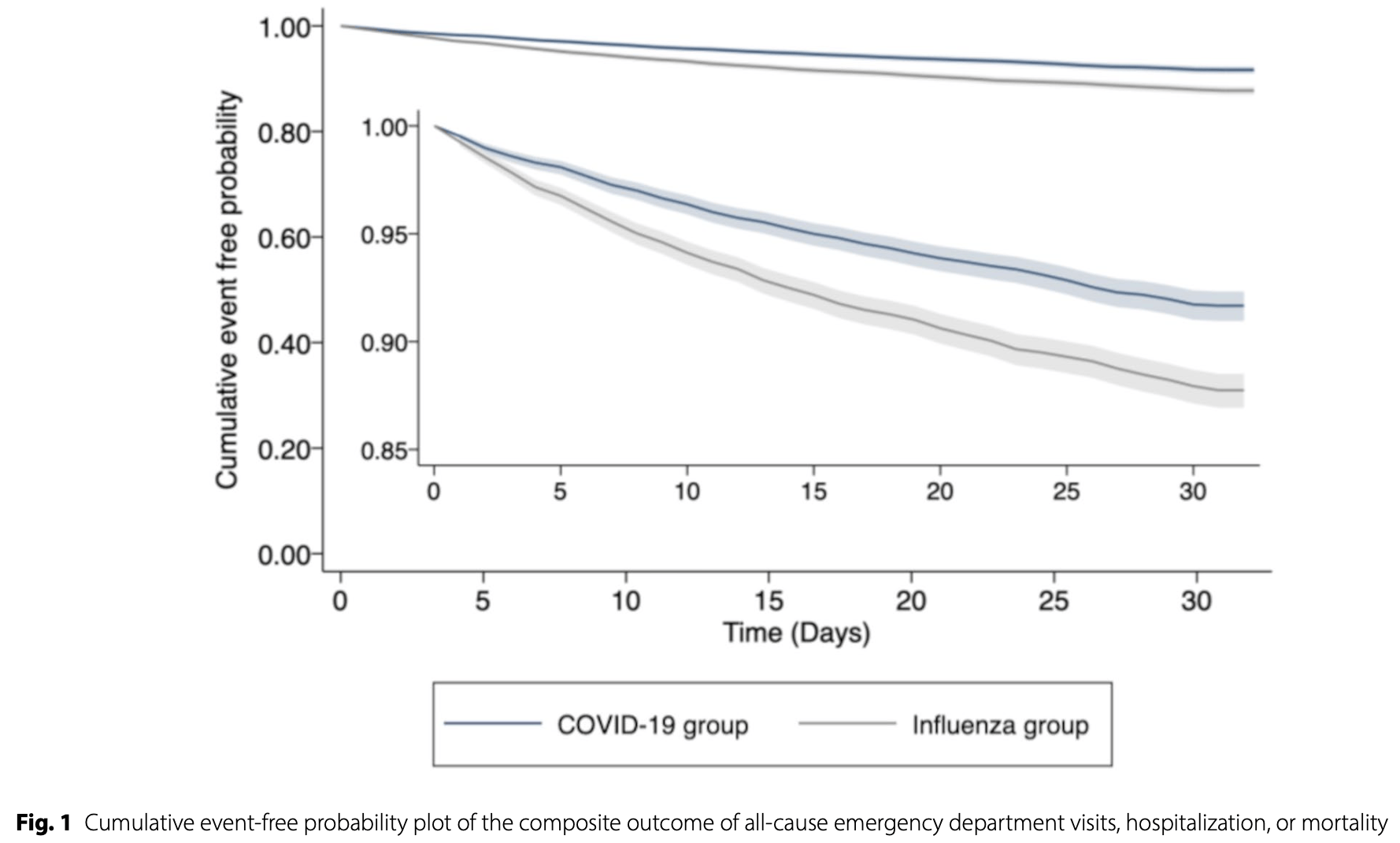
TriNetX retrospective 18,060 non-hospitalized patients showing lower risk of emergency department visits, hospitalization, and mortality in COVID-19 patients compared to influenza patients, with both groups treated with antivirals.
Hsu et al., 31 Mar 2025, retrospective, multiple countries, peer-reviewed, 8 authors, study period 1 October, 2022 - 31 January, 2023.
Contact: dtmed141@gmail.com.
Outcomes of non-hospitalized patients with COVID-19 versus seasonal influenza during the fall-winter 2022–2023 period
BMC Infectious Diseases, doi:10.1186/s12879-025-10833-6
Background The comparability of outcomes for non-hospitalized COVID-19 outpatients during the Omicron wave to outpatients with influenza remains uncertain. This study aims to compare the outcomes of non-hospitalized outpatients with COVID-19 and seasonal influenza during the fall-winter of 2022-2023. Methods This is a retrospective cohort study using TriNetX, a collaborative clinical research platform. Nonhospitalized outpatients with COVID-19 and seasonal influenza between 01 October 2022 and 31 January 2023 were selected from TriNetX. Propensity score matching (PSM) was used to compare patients receiving corresponding outpatient antiviral treatments. Hazard ratios (HRs) with 95% confidence intervals (CIs) for the primary outcome-a composite of all-cause emergency department (ED) visits, hospitalizations, or mortality during the 30-day follow-up period-were calculated and compared.
Results After PSM, two well-balanced groups of 9,030 patients each were identified. Non-hospitalized COVID-19 patients had a lower risk of primary composites outcomes including all-cause ED visits, hospitalization, or mortality (5.9% vs. 9.2%, HR, 0.661[95% CI, 0.593-0.737]) compared to the influenza group. In addition, the COVID-19 group demonstrated a reduced risk of all-cause ED visits (4.4% vs. 6.6%, HR 0.683[0.601-0.776]), hospitalization (1.7% vs. 2.9%, HR 0.605[0.495-0.739]) and mortality (0.1% vs. 0.2%, HR 0.176[0.052-0.597]), respectively.
Conclusions This study indicates a lower risk of all-cause ED visits, hospitalization, and mortality in the nonhospitalized COVID-19 patients compared to the seasonal influenza group, supporting the current public health strategy of adjusting COVID-19 management based on approaches used for seasonal influenza.
Abbreviations
Supplementary Information The online version contains supplementary material available at h t t p s : / / d o i . o r g / 1 0 . 1 1 8 6 / s 1 2 8 7 9 -0 2 5 -1 0 8 3 3 -6.
Supplementary Material 1 Author contributions WHH and CCL conceptualized the project. The study design was collaboratively developed by all contributing authors. Data collection was undertaken by BWS, YWT, JYW, THL, PYH, and MHC while data analysis and interpretation were a collective effort of all authors. WHH and CCL drafted the manuscript and all authors contributed to the critical revision of the manuscript, ensuring its intellectual rigor. The final manuscript received unanimous approval from all authors, who also jointly accepted the responsibility for the decision to submit it for publication.
Declarations Ethics approval and consent to participate The requirement for written informed consent was waived owing to the utilization of deidentified aggregate data. The study adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of Chi Mei Medical Center (approval number 11202-002).
Consent for publication Not required.
Competing interests All authors declared that there was no conflict of interest.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bhimraj, Morgan, Shumaker, Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19, Clin Infect Dis
Chen, Wei, Lai, Weng, Wang, A Meta-Analysis comparing the efficacy and safety of peramivir with other neuraminidase inhibitors for influenza treatment, Med
Chuang, Wu, Liu, Efficacy of nirmatrelvir and Ritonavir for postacute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection, J Med Virol
Dickow, Gunawardene, Willems, Higher in-hospital mortality in SARS-CoV-2 Omicron variant infection compared to influenza infection-Insights from the CORONA Germany study, PLoS ONE
Fung, Baye, Baik, Zheng, Mcdonald, Prevalence and characteristics of long COVID in elderly patients: an observational cohort study of over 2 million adults in the US, PLoS Med
Haukoos, Lewis, The propensity score, JAMA
Hedberg, Valik, Halim, Alfvén, Nauclér, Outcomes of SARS-CoV-2 Omicron variant infections compared with seasonal influenza and respiratory syncytial virus infections in adults attending the emergency department: A multicenter cohort study, Clin Infect Dis
Hsu, Shiau, Tsai, The effect of molnupiravir on post-acute outcome of COVID-19 survivors, J Infect
Hsu, Tsai, Wu, Liu, Lai, Post-acute hospitalization and mortality of nirmatrelvir plus Ritonavir for COVID-19 survivors, J Infect
Kopel, Bogdanov, Winer-Jones, Comparison of COVID-19 and Influenza-Related outcomes in the united States during Fall-Winter 2022-2023: A Cross-Sectional retrospective study, Diseases
Lai, Chao, Hsueh, Clinical efficacy of antiviral agents against coronavirus disease 2019: A systematic review of randomized controlled trials, J Microbiol Immunol Infect
Lai, Hsu, Yen, Long COVID: an inevitable sequela of SARS-CoV-2 infection, J Microbiol Immunol Infect
Lai, Wang, Wang, Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status, Int J Antimicrob Agents
Liu, Wu, Huang, Tsai, Lai, The effect of nirmatrelvir plus Ritonavir on the long-term risk of epilepsy and seizure following COVID-19: A retrospective cohort study including 91,528 patients, J Infect
Lo Re V 3rd, Dutcher, Connolly, Risk of admission to hospital with arterial or venous thromboembolism among patients diagnosed in the ambulatory setting with covid-19 compared with influenza: retrospective cohort study, BMJ Med
López-Medrano, Alfayate, Carratalà, Executive summary -Diagnosis, treatment and prophylaxis of influenza virus infection -Consensus statement of the Spanish society of infectious diseases and clinical microbiology (SEIMC), the Spanish society of pediatric infectious diseases (SEIP), the Spanish association of vaccinology (AEV), the Spanish society of family and community medicine (SEMFYC) and the Spanish society of preventive medicine, public health and health management (SEMPSPGS), Aten Primaria
Palchuk, London, Perez-Rey, A global federated real-world data and analytics platform for research, JAMIA Open
Shankar-Hari, Vale, Godolphin, Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A Meta-analysis, JAMA
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A Meta-analysis, JAMA
Topaloglu, Palchuk, Using a federated network of Real-World data to optimize clinical trials operations, JCO Clin Cancer Inf
Tsai, Tsai, Wu, The risk of methicillin-resistant Staphylococcus aureus infection following COVID-19 and influenza: A retrospective cohort study from the TriNetX network, J Infect
Uyeki, Bernstein, Bradley, Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa, Clin Infect Dis
Xie, Choi, Al-Aly, Risk of death in patients hospitalized for COVID-19 vs seasonal influenza in Fall-Winter 2022-2023, JAMA
DOI record:
{
"DOI": "10.1186/s12879-025-10833-6",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-025-10833-6",
"alternative-id": [
"10833"
],
"article-number": "442",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "17 September 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "19 March 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "31 March 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The requirement for written informed consent was waived owing to the utilization of deidentified aggregate data. The study adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of Chi Mei Medical Center (approval number 11202-002)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not required."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "All authors declared that there was no conflict of interest."
},
{
"group": {
"label": "Clinical trial number",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "not applicable."
}
],
"author": [
{
"affiliation": [],
"family": "Hsu",
"given": "Wan-Hsuan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shiau",
"given": "Bo-Wen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsai",
"given": "Ya-Wen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Jheng-Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Ting-Hui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Po-Yu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chuang",
"given": "Min-Hsiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lai",
"given": "Chih-Cheng",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
3,
31
]
],
"date-time": "2025-03-31T09:40:47Z",
"timestamp": 1743414047000
},
"deposited": {
"date-parts": [
[
2025,
3,
31
]
],
"date-time": "2025-03-31T09:41:27Z",
"timestamp": 1743414087000
},
"indexed": {
"date-parts": [
[
2025,
3,
31
]
],
"date-time": "2025-03-31T10:10:21Z",
"timestamp": 1743415821697,
"version": "3.40.3"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
3,
31
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
31
]
],
"date-time": "2025-03-31T00:00:00Z",
"timestamp": 1743379200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
31
]
],
"date-time": "2025-03-31T00:00:00Z",
"timestamp": 1743379200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-10833-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-025-10833-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-10833-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
3,
31
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
31
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "10833_CR1",
"unstructured": "World Health Organization. https://covid19.who.int/ Accessed on June 12, 2023."
},
{
"DOI": "10.1016/j.ijantimicag.2020.105946",
"author": "CC Lai",
"doi-asserted-by": "crossref",
"first-page": "105946",
"issue": "4",
"journal-title": "Int J Antimicrob Agents",
"key": "10833_CR2",
"unstructured": "Lai CC, Wang CY, Wang YH, et al. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55(4):105946.",
"volume": "55",
"year": "2020"
},
{
"key": "10833_CR3",
"unstructured": "Statement on the fifteenth meeting of the IHR. (2005) Emergency Committee on the COVID-19 pandemic [press release]. 5 May 2023."
},
{
"key": "10833_CR4",
"unstructured": "End of the Federal COVID-19. Public Health Emergency (PHE) Declaration [press release]. 5 May 2023."
},
{
"key": "10833_CR5",
"unstructured": "Ministry of Health LaW. Japan. Response to COVID 19 (Novel Coronavirus) after the classification change."
},
{
"key": "10833_CR6",
"unstructured": "Statement from Emer Cooke on the end. of the COVID-19 public health emergency [press release]. 8 May 2023."
},
{
"DOI": "10.1001/jama.2023.5348",
"author": "Y Xie",
"doi-asserted-by": "crossref",
"first-page": "1697",
"issue": "19",
"journal-title": "JAMA",
"key": "10833_CR7",
"unstructured": "Xie Y, Choi T, Al-Aly Z. Risk of death in patients hospitalized for COVID-19 vs seasonal influenza in Fall-Winter 2022–2023. JAMA. 2023;329(19):1697–9.",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1016/j.jmii.2022.10.003",
"author": "CC Lai",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "J Microbiol Immunol Infect",
"key": "10833_CR8",
"unstructured": "Lai CC, Hsu CK, Yen MY, et al. Long COVID: an inevitable sequela of SARS-CoV-2 infection. J Microbiol Immunol Infect. 2023;56(1):1–9.",
"volume": "56",
"year": "2023"
},
{
"author": "U Topaloglu",
"first-page": "1",
"journal-title": "JCO Clin Cancer Inf",
"key": "10833_CR9",
"unstructured": "Topaloglu U, Palchuk MB. Using a federated network of Real-World data to optimize clinical trials operations. JCO Clin Cancer Inf. 2018;2:1–10.",
"volume": "2",
"year": "2018"
},
{
"DOI": "10.1093/jamiaopen/ooad035",
"author": "MB Palchuk",
"doi-asserted-by": "crossref",
"first-page": "ooad035",
"issue": "2",
"journal-title": "JAMIA Open",
"key": "10833_CR10",
"unstructured": "Palchuk MB, London JW, Perez-Rey D, et al. A global federated real-world data and analytics platform for research. JAMIA Open. 2023;6(2):ooad035.",
"volume": "6",
"year": "2023"
},
{
"key": "10833_CR11",
"unstructured": "TriNetX. https://trinetx.com. Accessed June 25, 2023."
},
{
"DOI": "10.1016/j.jinf.2023.03.016",
"doi-asserted-by": "crossref",
"key": "10833_CR12",
"unstructured": "Hsu WH, Shiau BW, Tsai YW et al. The effect of molnupiravir on post-acute outcome of COVID-19 survivors. J Infect 2023."
},
{
"DOI": "10.1016/j.jinf.2023.02.007",
"author": "WH Hsu",
"doi-asserted-by": "crossref",
"first-page": "e107",
"issue": "4",
"journal-title": "J Infect",
"key": "10833_CR13",
"unstructured": "Hsu WH, Tsai YW, Wu JY, Liu TH, Lai CC. Post-acute hospitalization and mortality of nirmatrelvir plus Ritonavir for COVID-19 survivors. J Infect. 2023;86(4):e107–10.",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28750",
"author": "MH Chuang",
"doi-asserted-by": "crossref",
"first-page": "e28750",
"issue": "4",
"journal-title": "J Med Virol",
"key": "10833_CR14",
"unstructured": "Chuang MH, Wu JY, Liu TH, et al. Efficacy of nirmatrelvir and Ritonavir for post-acute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection. J Med Virol. 2023;95(4):e28750.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.jmii.2021.05.011",
"author": "CC Lai",
"doi-asserted-by": "crossref",
"first-page": "767",
"issue": "5",
"journal-title": "J Microbiol Immunol Infect",
"key": "10833_CR15",
"unstructured": "Lai CC, Chao CM, Hsueh PR. Clinical efficacy of antiviral agents against coronavirus disease 2019: A systematic review of randomized controlled trials. J Microbiol Immunol Infect. 2021;54(5):767–75.",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2023.01.006",
"author": "YW Tsai",
"doi-asserted-by": "crossref",
"first-page": "256",
"issue": "3",
"journal-title": "J Infect",
"key": "10833_CR16",
"unstructured": "Tsai YW, Tsai CF, Wu JY, et al. The risk of methicillin-resistant Staphylococcus aureus infection following COVID-19 and influenza: A retrospective cohort study from the TriNetX network. J Infect. 2023;86(3):256–308.",
"volume": "86",
"year": "2023"
},
{
"author": "TH Liu",
"first-page": "256",
"issue": "3",
"journal-title": "J Infect",
"key": "10833_CR17",
"unstructured": "Liu TH, Wu JY, Huang PY, Tsai YW, Lai CC. The effect of nirmatrelvir plus Ritonavir on the long-term risk of epilepsy and seizure following COVID-19: A retrospective cohort study including 91,528 patients. J Infect. 2023;86(3):256–308.",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1001/jama.2015.13480",
"author": "JS Haukoos",
"doi-asserted-by": "crossref",
"first-page": "1637",
"issue": "15",
"journal-title": "JAMA",
"key": "10833_CR18",
"unstructured": "Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637–8.",
"volume": "314",
"year": "2015"
},
{
"key": "10833_CR19",
"unstructured": "Bhimraj A, Morgan RL, Shumaker AH et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 2022."
},
{
"DOI": "10.1093/cid/ciy866",
"author": "TM Uyeki",
"doi-asserted-by": "crossref",
"first-page": "e1",
"issue": "6",
"journal-title": "Clin Infect Dis",
"key": "10833_CR20",
"unstructured": "Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis. 2019;68(6):e1–47.",
"volume": "68",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0292017",
"author": "J Dickow",
"doi-asserted-by": "crossref",
"first-page": "e0292017",
"issue": "9",
"journal-title": "PLoS ONE",
"key": "10833_CR21",
"unstructured": "Dickow J, Gunawardene MA, Willems S, et al. Higher in-hospital mortality in SARS-CoV-2 Omicron variant infection compared to influenza infection-Insights from the CORONA Germany study. PLoS ONE. 2023;18(9):e0292017.",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.3390/diseases12010016",
"doi-asserted-by": "crossref",
"key": "10833_CR22",
"unstructured": "Kopel H, Bogdanov A, Winer-Jones JP et al. Comparison of COVID-19 and Influenza-Related outcomes in the united States during Fall-Winter 2022–2023: A Cross-Sectional retrospective study. Diseases 2024;12(1)."
},
{
"key": "10833_CR23",
"unstructured": "National Institutes of Health. Information on COVID-19 treatment, prevention and research. COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/ Accessed April 15, 2023."
},
{
"DOI": "10.1001/jama.2021.11330",
"author": "M Shankar-Hari",
"doi-asserted-by": "crossref",
"first-page": "499",
"issue": "6",
"journal-title": "JAMA",
"key": "10833_CR24",
"unstructured": "Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A Meta-analysis. JAMA. 2021;326(6):499–518.",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"author": "JAC Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"issue": "13",
"journal-title": "JAMA",
"key": "10833_CR25",
"unstructured": "Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–41.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.3390/medicina56020063",
"doi-asserted-by": "crossref",
"key": "10833_CR26",
"unstructured": "Chen JY, Wei SK, Lai CC, Weng TS, Wang HH. A Meta-Analysis comparing the efficacy and safety of peramivir with other neuraminidase inhibitors for influenza treatment. Med (Kaunas) 2020;56(2)."
},
{
"DOI": "10.1016/j.aprim.2023.102629",
"author": "F López-Medrano",
"doi-asserted-by": "crossref",
"first-page": "102629",
"issue": "6",
"journal-title": "Aten Primaria",
"key": "10833_CR27",
"unstructured": "López-Medrano F, Alfayate S, Carratalà J, et al. Executive summary - Diagnosis, treatment and prophylaxis of influenza virus infection - Consensus statement of the Spanish society of infectious diseases and clinical microbiology (SEIMC), the Spanish society of pediatric infectious diseases (SEIP), the Spanish association of vaccinology (AEV), the Spanish society of family and community medicine (SEMFYC) and the Spanish society of preventive medicine, public health and health management (SEMPSPGS). Aten Primaria. 2023;55(6):102629.",
"volume": "55",
"year": "2023"
},
{
"DOI": "10.1136/bmjmed-2022-000421",
"author": "V Lo Re",
"doi-asserted-by": "crossref",
"first-page": "e000421",
"issue": "1",
"journal-title": "BMJ Med",
"key": "10833_CR28",
"unstructured": "Lo Re V 3rd, Dutcher SK, Connolly JG, et al. Risk of admission to hospital with arterial or venous thromboembolism among patients diagnosed in the ambulatory setting with covid-19 compared with influenza: retrospective cohort study. BMJ Med. 2023;2(1):e000421.",
"volume": "2",
"year": "2023"
},
{
"DOI": "10.1371/journal.pmed.1004194",
"author": "KW Fung",
"doi-asserted-by": "crossref",
"first-page": "e1004194",
"issue": "4",
"journal-title": "PLoS Med",
"key": "10833_CR29",
"unstructured": "Fung KW, Baye F, Baik SH, Zheng Z, McDonald CJ. Prevalence and characteristics of long COVID in elderly patients: an observational cohort study of over 2 million adults in the US. PLoS Med. 2023;20(4):e1004194.",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciad660",
"author": "P Hedberg",
"doi-asserted-by": "crossref",
"first-page": "900",
"issue": "4",
"journal-title": "Clin Infect Dis",
"key": "10833_CR30",
"unstructured": "Hedberg P, Karlsson Valik J, Abdel-Halim L, Alfvén T, Nauclér P. Outcomes of SARS-CoV-2 Omicron variant infections compared with seasonal influenza and respiratory syncytial virus infections in adults attending the emergency department: A multicenter cohort study. Clin Infect Dis. 2024;78(4):900–7.",
"volume": "78",
"year": "2024"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-025-10833-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Outcomes of non-hospitalized patients with COVID-19 versus seasonal influenza during the fall-winter 2022–2023 period",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}