Nov 3 2022 |
et al., Evidence-Based Complementary and Alternative Medicine, doi:10.1155/2022/3125662 | Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study |
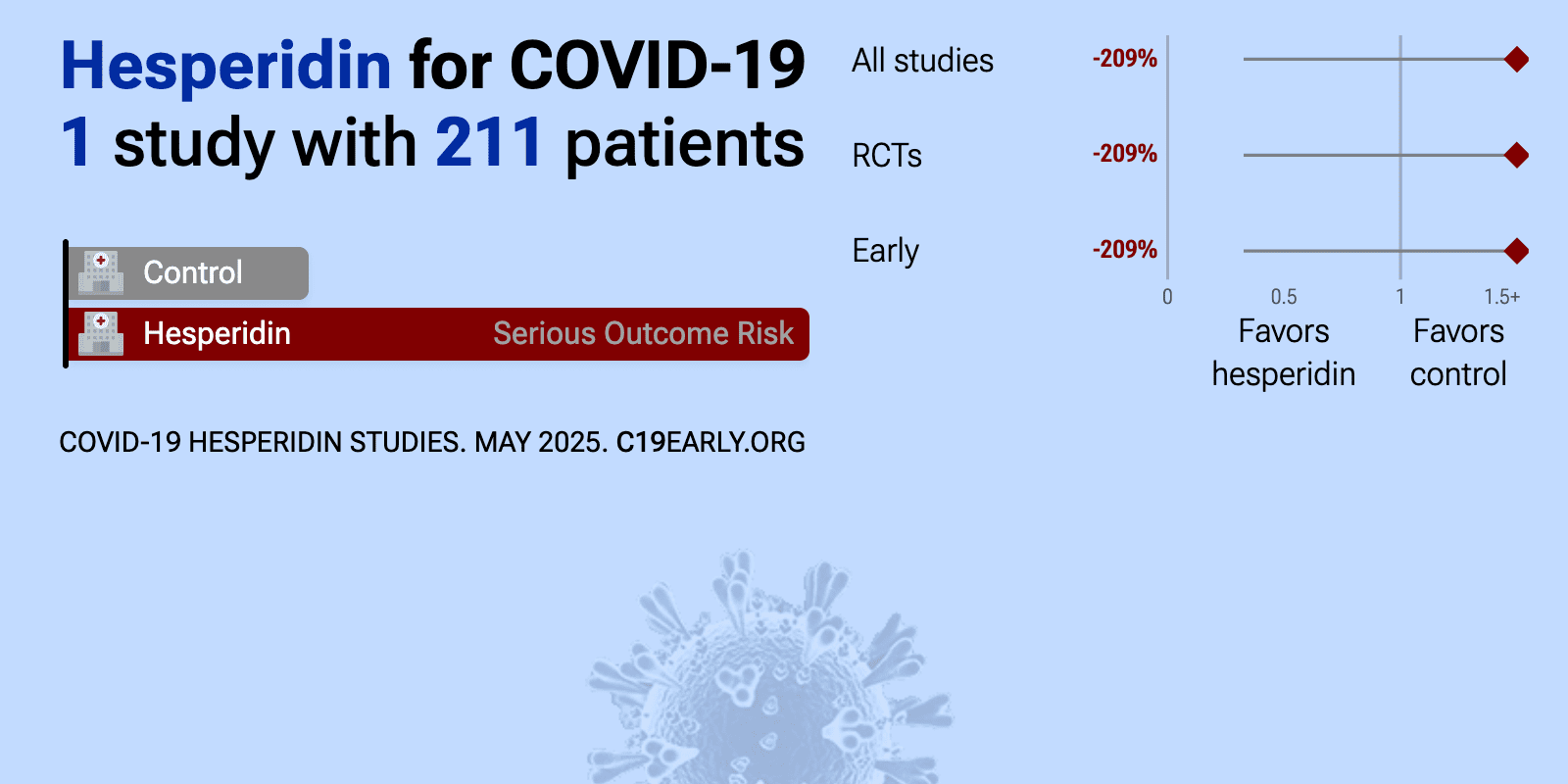
| 209% higher progression (p=0.36) and 31% improved recovery (p=0.23). RCT 216 COVID-19 outpatients showing no significant difference in primary outcome (presence of fever, cough, shortness of breath, or anosmia) with hesperidin treatment versus placebo after 14 days, though a post-hoc sensitivity analysis s.. | ||
Oct 30 2020 |
et al., NCT04452799 | Randomized Double-blind Controlled Parallel Study of (Hesperidin and Diosmin Mixture) for Treatment of COVID-19 Newly Diagnosed Patients in Egypt |
| Estimated 100 patient hesperidin early treatment RCT with results not reported over 5 years after estimated completion. | ||