Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study
et al., Evidence-Based Complementary and Alternative Medicine, doi:10.1155/2022/3125662, NCT04715932, Nov 2022
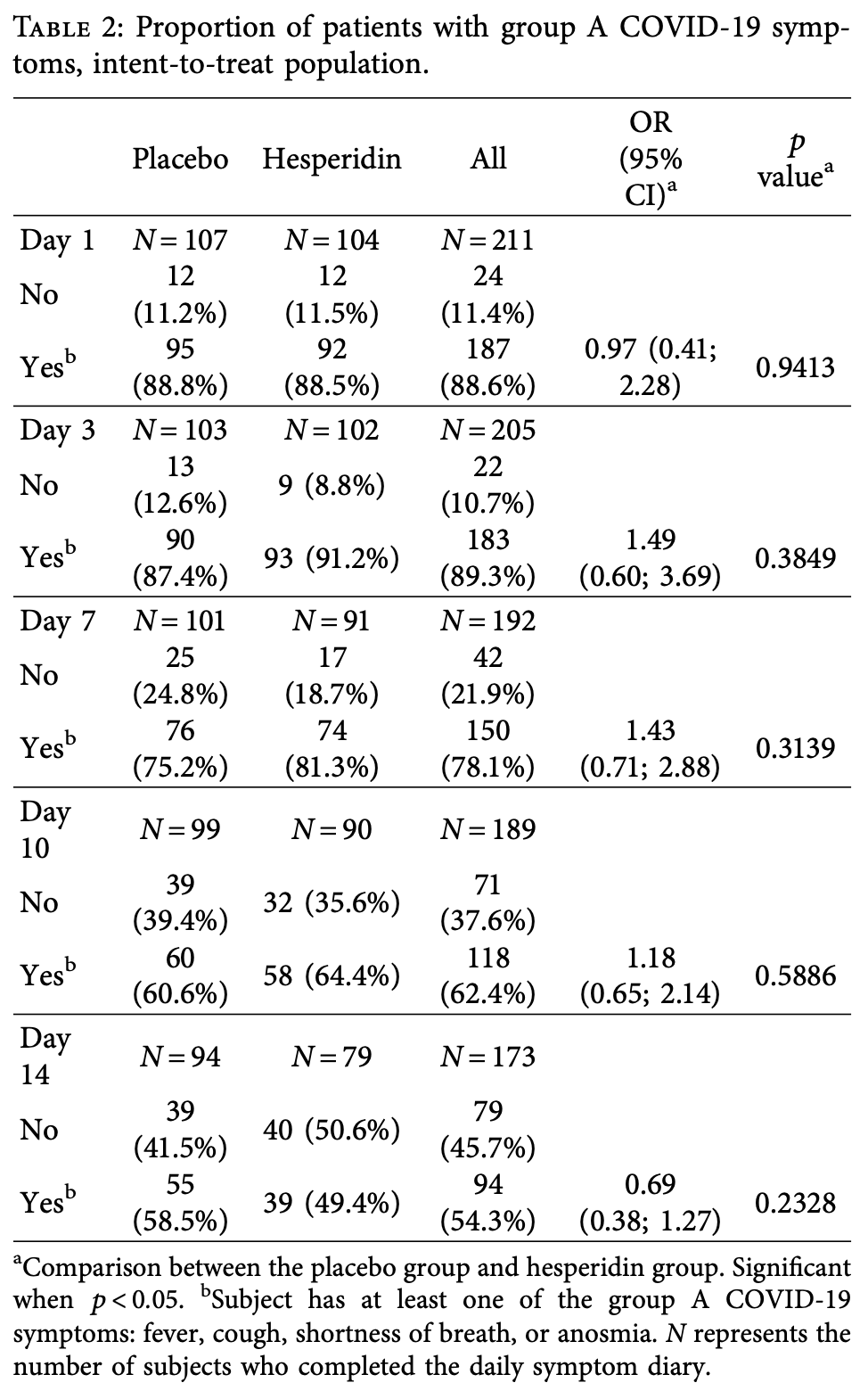
RCT 216 COVID-19 outpatients showing no significant difference in primary outcome (presence of fever, cough, shortness of breath, or anosmia) with hesperidin treatment versus placebo after 14 days, though a post-hoc sensitivity analysis showed significant benefit. Authors suggest that earlier treatment and higher dosage may show greater benefit in future studies.
|
risk of progression, 208.7% higher, RR 3.09, p = 0.36, treatment 3 of 104 (2.9%), control 1 of 107 (0.9%), hospitalization, ventilation, or death.
|
|
risk of no recovery, 31.0% lower, OR 0.69, p = 0.23, treatment 104, control 107, group A symptoms, day 14, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Dupuis et al., 3 Nov 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Canada, peer-reviewed, mean age 41.0, 11 authors, study period 18 February, 2021 - 20 May, 2021, trial NCT04715932 (history).
Contact: dupuisj@icloud.com.
Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study
Evidence-Based Complementary and Alternative Medicine, doi:10.1155/2022/3125662
COVID-19 symptoms can cause substantial disability, yet no therapy can currently reduce their frequency or duration. We conducted a double-blind placebo-controlled trial of hesperidin 1000 mg once daily for 14 days in 216 symptomatic nonvaccinated COVID-19 subjects. Tirteen symptoms were recorded after 3, 7, 10, and 14 days. Te primary endpoint was the proportion of subjects with any of four cardinal (group A) symptoms: fever, cough, shortness of breath, or anosmia. At the baseline, symptoms in decreasing frequency were as follows: cough (53.2%), weakness (44.9%), headache (42.6%), pain (35.2%), sore throat (28.7%), runny nose (26.9%), chills (22.7%), shortness of breath (22.2%), anosmia (18.5%), fever (16.2%), diarrhea (6.9%), nausea/vomiting (6.5%), and irritability/confusion (3.2%). Group A symptoms in the placebo vs. hesperidin group were 88.8% vs. 88.5% (day 1) and reduced to 58.5 vs. 49.4% at day 14 (OR 0.69, 95% CI 0.38-1.27, p � 0.23). At day 14, 15 subjects in the placebo group and 28 in the hesperidin group failed to report their symptoms. In an attrition bias analysis imputing "no symptoms" to missing values, the hesperidin group showed reduction of 14.5% of group A symptoms from 50.9% to 36.4% (OR: 0.55, 0.32-0.96, p � 0.03). Anosmia, the most frequent persisting symptom (29.3%), was lowered by 7.3% to 25.3% in the hesperidin group vs. 32.6% in the placebo group (p � 0.29). Te mean number of symptoms in the placebo and hesperidin groups was 5.10 (SD 2.26) vs. 5.48 (SD 2.35) (day 1) and 1.40 (SD 1.65) vs. 1.38 (SD 1.76) (day 14) (p � 0.92). In conclusion, most nonvaccinated COVID-19 infected subjects remain symptomatic after 14 days with anosmia being the most frequently persisting symptom. Hesperidin 1 g daily may help reduce group A symptoms. Earlier treatment of longer duration and/or higher dosage should be tested.
Disclosure Te other funders did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
References
Bellavite, Donzelli, Hesperidin and SARS-CoV-2: new light on the healthy function of citrus fruits, Antioxidants
Beltran-Corbellini, Chico-Garcia, Martinez-Poles, Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study, European Journal of Neurology
Chanet, Milenkovic, Manach, Mazur, Morand, Citrus favanones: what is their role in cardiovascular protection?, Journal of Agricultural and Food Chemistry
Demonty, Lin, Zebregs, Te citrus favonoids hesperidin and naringin do not afect serum cholesterol in moderately hypercholesterolemic men and women, Journal of Nutrition
Dennis, GRAS notice (GRN) no. 901
Ding, Sun, Zhu, Hesperidin attenuates infuenza A virus (H1N1) induced lung injury in rats through its antiinfammatory efect, Antiviral Terapy
Dupuis, Laurin, Tardif, Fourteen-days evolution of COVID-19 symptoms during the third wave in non-vaccinated subjects and efects of hesperidin therapy: a randomized, double-blinded, placebo-controlled study
Fu, Wang, Yuan, Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis, Journal of Infection
Haggag, El-Ashmawy, Okasha, Is hesperidin essential for prophylaxis and treatment of COVID-19 infection?, Medical Hypotheses
Hajialyani, Hosein, Farzaei, Echeverria, Nabavi et al., Hesperidin as a neuroprotective agent: a review of animal and clinical evidence, Molecules
He, Lau, Wu, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nature Medicine
Hofmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Te Lancet
Lacroix, Beau, Hurault-Delarue, First epidemiological data for venotonics in pregnancy from the EFEMERIS database, Phlebology
Li, Huang, Zou, Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes, Journal of Medical Virology
Li, Kandhare, Mukherjee, Bodhankar, Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats, Regulatory Toxicology and Pharmacology
Lin, Tsai, Tsai, Anti-SARS coronavirus 3C-like protease efects of Isatis indigotica root and plantderived phenolic compounds, Antiviral Research
Liu, Li, Xu, Prognostic value of interleukin-6, Creactive protein, and procalcitonin in patients with COVID-19, Journal of Clinical Virology
Ma, Feng, Ding, Hesperetin attenuates ventilator-induced acute lung injury through inhibition of Evidence-Based Complementary and Alternative Medicine NF-κB-mediated infammation, European Journal of Pharmacology
Mizrahi, Shilo, Rossman, Longitudinal symptom dynamics of COVID-19 infection, Nature Communications
Moein, Hashemian, Mansourafshar, Khorram-Tousi, Tabarsi et al., Smell dysfunction: a biomarker for COVID-19, International Forum of Allergy & Rhinology
Rizza, Muniyappa, Iantorno, Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing infammatory markers in patients with metabolic syndrome, Journal of Clinical Endocrinology and Metabolism
Susan, GRAS notice (GRN) no. 796
Tenforde, Kim, Lindsell, Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network-United States, March-June 2020, Morbidity and Mortality Weekly Report
Testai, Calderone, Nutraceutical value of citrus favanones and their implications in cardiovascular disease, Nutrients
Wu, Liu, Yang, Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharmaceutica Sinica B
Ye, Guan, Lu, Protective efects of hesperetin on lipopolysaccharide-induced acute lung injury by targeting MD2, European Journal of Pharmacology
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, New England Journal of Medicine
DOI record:
{
"DOI": "10.1155/2022/3125662",
"ISSN": [
"1741-4288",
"1741-427X"
],
"URL": "http://dx.doi.org/10.1155/2022/3125662",
"abstract": "<jats:p>COVID-19 symptoms can cause substantial disability, yet no therapy can currently reduce their frequency or duration. We conducted a double-blind placebo-controlled trial of hesperidin 1000 mg once daily for 14 days in 216 symptomatic nonvaccinated COVID-19 subjects. Thirteen symptoms were recorded after 3, 7, 10, and 14 days. The primary endpoint was the proportion of subjects with any of four cardinal (group A) symptoms: fever, cough, shortness of breath, or anosmia. At the baseline, symptoms in decreasing frequency were as follows: cough (53.2%), weakness (44.9%), headache (42.6%), pain (35.2%), sore throat (28.7%), runny nose (26.9%), chills (22.7%), shortness of breath (22.2%), anosmia (18.5%), fever (16.2%), diarrhea (6.9%), nausea/vomiting (6.5%), and irritability/confusion (3.2%). Group A symptoms in the placebo vs. hesperidin group were 88.8% vs. 88.5% (day 1) and reduced to 58.5 vs. 49.4% at day 14 (OR 0.69, 95% CI 0.38–1.27, <jats:inline-formula>\n <a:math xmlns:a=\"http://www.w3.org/1998/Math/MathML\" id=\"M1\">\n <a:mi>p</a:mi>\n <a:mo>=</a:mo>\n <a:mn>0.23</a:mn>\n </a:math>\n </jats:inline-formula>). At day 14, 15 subjects in the placebo group and 28 in the hesperidin group failed to report their symptoms. In an attrition bias analysis imputing “no symptoms” to missing values, the hesperidin group showed reduction of 14.5% of group A symptoms from 50.9% to 36.4% (OR: 0.55, 0.32–0.96, <jats:inline-formula>\n <c:math xmlns:c=\"http://www.w3.org/1998/Math/MathML\" id=\"M2\">\n <c:mi>p</c:mi>\n <c:mo>=</c:mo>\n <c:mn>0.03</c:mn>\n </c:math>\n </jats:inline-formula>). Anosmia, the most frequent persisting symptom (29.3%), was lowered by 7.3% to 25.3% in the hesperidin group vs. 32.6% in the placebo group (<jats:inline-formula>\n <e:math xmlns:e=\"http://www.w3.org/1998/Math/MathML\" id=\"M3\">\n <e:mi>p</e:mi>\n <e:mo>=</e:mo>\n <e:mn>0.29</e:mn>\n </e:math>\n </jats:inline-formula>). The mean number of symptoms in the placebo and hesperidin groups was 5.10 (SD 2.26) vs. 5.48 (SD 2.35) (day 1) and 1.40 (SD 1.65) vs. 1.38 (SD 1.76) (day 14) (<jats:inline-formula>\n <g:math xmlns:g=\"http://www.w3.org/1998/Math/MathML\" id=\"M4\">\n <g:mi>p</g:mi>\n <g:mo>=</g:mo>\n <g:mn>0.92</g:mn>\n </g:math>\n </jats:inline-formula>). In conclusion, most nonvaccinated COVID-19 infected subjects remain symptomatic after 14 days with anosmia being the most frequently persisting symptom. Hesperidin 1 g daily may help reduce group A symptoms. Earlier treatment of longer duration and/or higher dosage should be tested.</jats:p>",
"alternative-id": [
"3125662",
"3125662"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-3193-1014",
"affiliation": [
{
"name": "Montreal Heart Institute Research Center, Montreal, Quebec, Canada"
},
{
"name": "Department of Medicine of Université de Montréal, Montreal, Quebec, Canada"
}
],
"authenticated-orcid": true,
"family": "Dupuis",
"given": "Jocelyn",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Ingenew Pharmaceuticals, Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Laurin",
"given": "Pierre",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8200-8983",
"affiliation": [
{
"name": "Montreal Heart Institute Research Center, Montreal, Quebec, Canada"
},
{
"name": "Department of Medicine of Université de Montréal, Montreal, Quebec, Canada"
}
],
"authenticated-orcid": true,
"family": "Tardif",
"given": "Jean-Claude",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Montreal Heart Institute Research Center, Montreal, Quebec, Canada"
}
],
"family": "Hausermann",
"given": "Leslie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Rosa",
"given": "Camille",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Guertin",
"given": "Marie-Claude",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ingenew Pharmaceuticals, Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Thibaudeau",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ingenew Pharmaceuticals, Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Gagnon",
"given": "Lyne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ingenew Pharmaceuticals, Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Cesari",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ingenew Pharmaceuticals, Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Robitaille",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ingenew Pharmaceuticals, Montreal Health Innovations Coordination Center, Montreal, Quebec, Canada"
}
],
"family": "Moran",
"given": "John E.",
"sequence": "additional"
}
],
"container-title": "Evidence-Based Complementary and Alternative Medicine",
"container-title-short": "Evidence-Based Complementary and Alternative Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T17:20:08Z",
"timestamp": 1667496008000
},
"deposited": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T17:20:13Z",
"timestamp": 1667496013000
},
"editor": [
{
"affiliation": [],
"family": "Adnan",
"given": "Mohd",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/501100012679",
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100012679",
"id-type": "DOI"
}
],
"name": "Montreal Heart Institute"
},
{
"name": "Valeo Pharma Inc"
}
],
"indexed": {
"date-parts": [
[
2025,
5,
14
]
],
"date-time": "2025-05-14T02:45:57Z",
"timestamp": 1747190757953,
"version": "3.40.5"
},
"is-referenced-by-count": 11,
"issued": {
"date-parts": [
[
2022,
11,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T00:00:00Z",
"timestamp": 1667433600000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/ecam/2022/3125662.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ecam/2022/3125662.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ecam/2022/3125662.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1-10",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2022,
11,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
11,
3
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "1"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "2"
},
{
"DOI": "10.1002/jmv.26424",
"doi-asserted-by": "publisher",
"key": "3"
},
{
"DOI": "10.1111/ene.14273",
"doi-asserted-by": "publisher",
"key": "4"
},
{
"DOI": "10.1002/alr.22587",
"doi-asserted-by": "publisher",
"key": "5"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "6"
},
{
"DOI": "10.1016/j.jcv.2020.104370",
"doi-asserted-by": "publisher",
"key": "7"
},
{
"DOI": "10.1016/j.antiviral.2005.07.002",
"doi-asserted-by": "publisher",
"key": "8"
},
{
"DOI": "10.1016/j.apsb.2020.02.008",
"doi-asserted-by": "publisher",
"key": "9"
},
{
"DOI": "10.3851/IMP3235",
"doi-asserted-by": "publisher",
"key": "10"
},
{
"DOI": "10.1016/j.ejphar.2015.11.038",
"doi-asserted-by": "publisher",
"key": "11"
},
{
"DOI": "10.1016/j.ejphar.2019.02.042",
"doi-asserted-by": "publisher",
"key": "12"
},
{
"DOI": "10.3390/molecules24030648",
"doi-asserted-by": "publisher",
"key": "13"
},
{
"DOI": "10.1021/jf300669s",
"doi-asserted-by": "publisher",
"key": "14"
},
{
"DOI": "10.3390/nu9050502",
"doi-asserted-by": "publisher",
"key": "15"
},
{
"DOI": "10.15585/mmwr.mm6930e1",
"doi-asserted-by": "publisher",
"key": "16"
},
{
"DOI": "10.1016/j.jinf.2020.03.041",
"doi-asserted-by": "publisher",
"key": "17"
},
{
"DOI": "10.1038/s41467-020-20053-y",
"doi-asserted-by": "publisher",
"key": "18"
},
{
"DOI": "10.3390/antiox9080742",
"doi-asserted-by": "publisher",
"key": "19"
},
{
"DOI": "10.1016/j.mehy.2020.109957",
"doi-asserted-by": "publisher",
"key": "20"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"doi-asserted-by": "publisher",
"key": "21"
},
{
"DOI": "10.1016/j.yrtph.2019.04.001",
"doi-asserted-by": "publisher",
"key": "22"
},
{
"DOI": "10.1210/jc.2010-2879",
"doi-asserted-by": "publisher",
"key": "23"
},
{
"DOI": "10.3945/jn.110.124735",
"doi-asserted-by": "publisher",
"key": "24"
},
{
"DOI": "10.1177/0268355515589679",
"doi-asserted-by": "publisher",
"key": "25"
},
{
"article-title": "GRAS notice (GRN) no. 796 [internet]",
"author": "C. Susan",
"key": "26",
"year": "2018"
},
{
"article-title": "GRAS notice (GRN) no. 901 [internet]",
"author": "M. K. Dennis",
"key": "27",
"year": "2019"
},
{
"article-title": "Fourteen-days evolution of COVID-19 symptoms during the third wave in non-vaccinated subjects and effects of hesperidin therapy: a randomized, double-blinded, placebo-controlled study",
"author": "J. Dupuis",
"journal-title": "medRxiv",
"key": "28",
"year": "2021"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/ecam/2022/3125662/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study",
"type": "journal-article",
"volume": "2022"
}