Long-term follow-up of treatment comparisons in RECOVERY: a randomised, open-label, platform trial for patients hospitalised with COVID-19
et al., medRxiv, doi:10.1101/2025.08.29.25334732, NCT04381936, Sep 2025
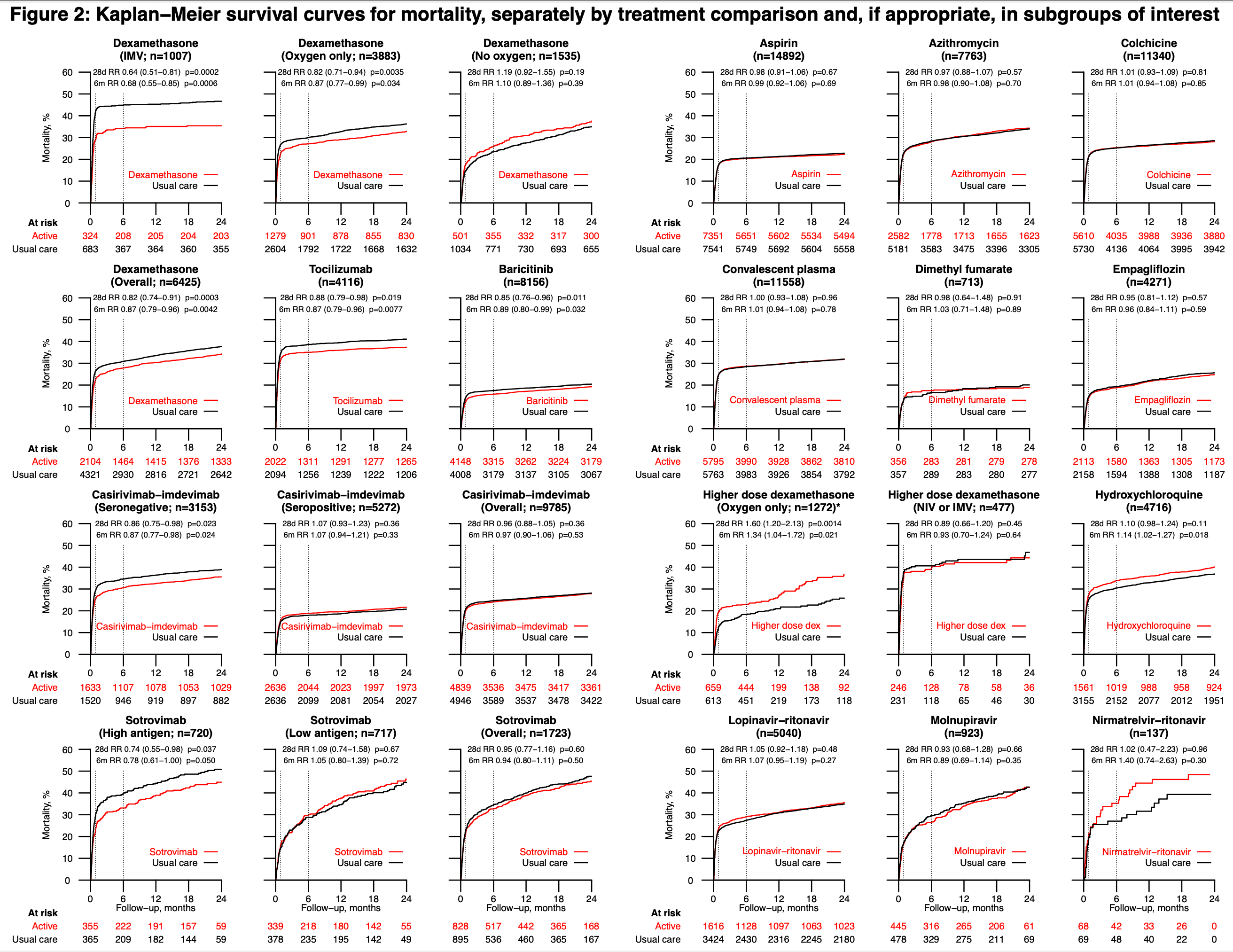
6-month followup of RECOVERY patients. Results are reported within the respective trials for each treatment.
Horby et al., 2 Sep 2025, Randomized Controlled Trial, preprint, 38 authors, trial NCT04381936 (history).
Contact: recoverytrial@ndph.ox.ac.uk.
Long-term follow-up of treatment comparisons in RECOVERY: a randomised, open-label, platform trial for patients hospitalised with COVID-19
doi:10.1101/2025.08.29.25334732
Background: The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial evaluated the effects of sixteen potential treatments for patients hospitalised with COVID-19. Dexamethasone (at a dose of 6mg daily), tocilizumab, baricitinib, and the monoclonal antibodies casirivimab-imdevimab and sotrovimab were shown to reduce 28-day mortality in all or specific groups of patients. Here we report the long-term efficacy and safety of all sixteen therapies. Methods: Patients hospitalised with COVID-19 were potentially eligible to join this randomised, controlled, open-label, platform trial. Participants were randomly allocated to receive each trial treatment, or not, on top of usual care. Analyses were by intention to treat comparing each treatment with its own usual care control group. The pre-specified primary long-term follow-up outcome was 6-month all-cause mortality, presented as mortality rate ratios adjusted for baseline age and ventilation status. The key safety outcomes were major non-COVID infection and non-COVID death at 6 months. ISRCTN50189673 and NCT04381936. Findings: Between 19 March 2020 and 19 March 2024, 48,402 patients were included in RECOVERY COVID-19 treatment comparisons. For each of the treatments previously demonstrated to be effective at 28 days, the early mortality benefit was preserved up to 6 months. Among 6425 patients in the dexamethasone (6mg daily) comparison, 6-month mortality was 34.3% vs 44.4% in the invasive mechanical ventilation group (rate ratio [RR] 0.68; 95% confidence interval [CI] 0.55-0.85; p=0.0006); 27.7% vs 29.2% in the oxygen or non-invasive ventilation group (RR 0.87; 95% CI 0.77-0.99; p=0.034); and 26.1% vs 22.5% in the no oxygen group (RR 1.10; 95% CI 0.89-1.36; p=0.39); test for trend p=0.0024. Among 4116 patients in the . tocilizumab comparison, 34.3% vs 38.9% died within 6 months (RR 0.87; 95% CI 0.79-0.96; p=0.0077). Among 8156 patients in the baricitinib comparison, 15.7% vs. 16.6% died (RR 0.89; 95% CI 0.80-0.99; p=0.032). Among 3153 anti-SARS-CoV-2 serum antibody negative patients (the primary analysis population) in the casirivimabimdevimab comparison, 29.3% vs. 34.7% died (RR 0.87; 95% CI 0.77-0.98; p=0.024). Among 720 patients with high serum nucleocapsid antigen concentration (the primary analysis population) in the sotrovimab comparison, 33.0% vs. 38.6% died (RR 0.78; 95% CI 0.61-1.00; p=0.050). In line with the 28-day results, aspirin, azithromycin, colchicine, convalescent plasma, dimethyl fumarate, empagliflozin, lopinavir-ritonavir, molnupiravir, and nirmatrelvir-ritonavir did not reduce 6-month mortality. Mortality at 6 months was higher with hydroxychloroquine therapy (33.3% vs. 30.2%; RR 1.14; 95% CI 1.02-1.27; p=0.018) and, among hypoxic patients not requiring ventilatory support, with higher dose dexamethasone (initial dose 20mg daily, 22.0% vs. 17.6%, RR 1.34; 95% CI 1.04-1.72; p=0.021). Allocation to dexamethasone 6mg once daily resulted in a small increase in major non-COVID..
RECOVERY long-term follow-up
Declaration of interests The authors have no conflict of interest or financial relationships relevant to the submitted work to disclose. No form of payment was given to anyone to produce the manuscript. The Nuffield Department of Population Health at the University of Oxford has a staff policy of not accepting honoraria or consultancy fees directly or indirectly
References
Abani, Abbas, Abbas, Higher dose corticosteroids in hospitalised COVID-19 patients requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial, eClinicalMedicine, doi:10.1016/j.eclinm.2025.103080
Agarwal, Hunt, Stegemann, A living WHO guideline on drugs for covid-19, BMJ
Alderson, Roberts, Corticosteroids for acute traumatic brain injury, Cochrane Database Syst Rev
Annane, Bellissant, Bollaert, Corticosteroids for treating sepsis in children and adults, Cochrane Database Syst Rev
Baghdadi, Coffey, Adediran, Antibiotic Use and Bacterial Infection among Inpatients in the First Wave of COVID-19: a Retrospective Cohort Study of 64,691 Patients, Antimicrob Agents Chemother
Bieber, Feist, Irvine, A Review of Safety Outcomes from Clinical Trials of Baricitinib in Rheumatology, Dermatology and COVID-19, Adv Ther
Cain, Cidlowski, Immune regulation by glucocorticoids, Nat Rev Immunol
Group, Horby, Mafham, Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19, N Engl J Med
Group, Horby, Peto, Dimethyl fumarate in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Nat Commun
Group, Horby, Staplin, Molnupiravir or nirmatrelvir-ritonavir versus usual care in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label
Guaraldi, Meschiari, Cozzi-Lepri, Tocilizumab in patients with severe COVID-19: a retrospective cohort study, Lancet Rheumatol
Higgins, Berry, Long-term (180-Day) Outcomes in Critically Ill Patients With COVID-19 in the REMAP-CAP Randomized Clinical Trial, JAMA
Horby, Emberson, Peto, Sotrovimab versus usual care in patients admitted to hospital with COVID-19: a randomised, controlled, open-label, platform trial, RECOVERY)
Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med
Musuuza, Watson, Parmasad, Putman-Buehler, Christensen et al., Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis, PLoS One
Pessoa-Amorim, Campbell, Fletcher, Making trials part of good clinical care: lessons from the RECOVERY trial, Future Healthc J
Ramzi, Hospital readmissions and post-discharge all-cause mortality in COVID-19 recovered patients; A systematic review and meta-analysis, Am J Emerg Med
Recovery Collaborative, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Recovery Collaborative, Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Recovery Collaborative, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet Respir Med
Recovery Collaborative, Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Shankar-Hari, Vale, Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Metaanalysis, JAMA
Singh, Beg, Lopez-Olivo, Tocilizumab for rheumatoid arthritis, Cochrane Database Syst Rev
Stern, Skalsky, Avni, Carrara, Leibovici et al., Corticosteroids for pneumonia, Cochrane Database Syst Rev
Stuck, Minder, Frey, Risk of infectious complications in patients taking glucocorticosteroids, Rev Infect Dis
Trøseid, Arribas, Assoumou, Efficacy and safety of baricitinib in hospitalized adults with severe or critical COVID-19 (Bari-SolidAct): a randomised, double-blind, placebo-controlled phase 3 trial, Dexamethasone IMV
Wang, Sun, Wang, Efficacy and Safety of Tofacitinib, Baricitinib, and Upadacitinib for Rheumatoid Arthritis: A Systematic Review and Meta-Analysis, Mayo Clin Proc
DOI record:
{
"DOI": "10.1101/2025.08.29.25334732",
"URL": "http://dx.doi.org/10.1101/2025.08.29.25334732",
"abstract": "<jats:p>Background: The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial evaluated the effects of sixteen potential treatments for patients hospitalised with COVID-19. Dexamethasone (at a dose of 6mg daily), tocilizumab, baricitinib, and the monoclonal antibodies casirivimab-imdevimab and sotrovimab were shown to reduce 28-day mortality in all or specific groups of patients. Here we report the long-term efficacy and safety of all sixteen therapies.\n\nMethods: Patients hospitalised with COVID-19 were potentially eligible to join this randomised, controlled, open-label, platform trial. Participants were randomly allocated to receive each trial treatment, or not, on top of usual care. Analyses were by intention to treat comparing each treatment with its own usual care control group. The pre-specified primary long-term follow-up outcome was 6-month all-cause mortality, presented as mortality rate ratios adjusted for baseline age and ventilation status. The key safety outcomes were major non-COVID infection and non-COVID death at 6 months. <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"isrctn\" xlink:href=\"50189673\">ISRCTN50189673</jats:ext-link> and <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04381936\">NCT04381936</jats:ext-link>.\n\nFindings: Between 19 March 2020 and 19 March 2024, 48,402 patients were included in RECOVERY COVID-19 treatment comparisons. For each of the treatments previously demonstrated to be effective at 28 days, the early mortality benefit was preserved up to 6 months. Among 6425 patients in the dexamethasone (6mg daily) comparison, 6-month mortality was 34.3% vs 44.4% in the invasive mechanical ventilation group (rate ratio [RR] 0.68; 95% confidence interval [CI] 0.55-0.85; p=0.0006); 27.7% vs 29.2% in the oxygen or non-invasive ventilation group (RR 0.87; 95% CI 0.77-0.99; p=0.034); and 26.1% vs 22.5% in the no oxygen group (RR 1.10; 95% CI 0.89-1.36; p=0.39); test for trend p=0.0024. Among 4116 patients in the tocilizumab comparison, 34.3% vs 38.9% died within 6 months (RR 0.87; 95% CI 0.79-0.96; p=0.0077). Among 8156 patients in the baricitinib comparison, 15.7% vs. 16.6% died (RR 0.89; 95% CI 0.80-0.99; p=0.032). Among 3153 anti-SARS-CoV-2 serum antibody negative patients (the primary analysis population) in the casirivimab-imdevimab comparison, 29.3% vs. 34.7% died (RR 0.87; 95% CI 0.77-0.98; p=0.024). Among 720 patients with high serum nucleocapsid antigen concentration (the primary analysis population) in the sotrovimab comparison, 33.0% vs. 38.6% died (RR 0.78; 95% CI 0.61-1.00; p=0.050). In line with the 28-day results, aspirin, azithromycin, colchicine, convalescent plasma, dimethyl fumarate, empagliflozin, lopinavir-ritonavir, molnupiravir, and nirmatrelvir-ritonavir did not reduce 6-month mortality. Mortality at 6 months was higher with hydroxychloroquine therapy (33.3% vs. 30.2%; RR 1.14; 95% CI 1.02-1.27; p=0.018) and, among hypoxic patients not requiring ventilatory support, with higher dose dexamethasone (initial dose 20mg daily, 22.0% vs. 17.6%, RR 1.34; 95% CI 1.04-1.72; p=0.021). Allocation to dexamethasone 6mg once daily resulted in a small increase in major non-COVID infection within 6 months compared with usual care (21.4% vs. 19.1%; absolute difference 2.2%; 95% CI 0.2-4.5%). We found no evidence that any other treatments increased the risk of major non-COVID infection.\n\nInterpretation: In patients hospitalised with COVID-19, dexamethasone (at a dose of 6mg daily in hypoxic patients), tocilizumab (in hypoxic patients with CRP ≥75 mg/L), baricitinib, casirivimab-imdevimab (in seronegative patients), and sotrovimab (in high antigen patients) reduced 6-month mortality. Dexamethasone at a dose of 6mg daily was associated with an increase in major non-COVID infection but there was no evidence of other later emerging harms. Other treatments tested in RECOVERY did not reduce 6-month mortality.</jats:p>",
"accepted": {
"date-parts": [
[
2025,
9,
2
]
]
},
"author": [
{
"ORCID": "https://orcid.org/0000-0002-9822-1586",
"affiliation": [],
"authenticated-orcid": false,
"family": "Horby",
"given": "Peter W",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-4100-2163",
"affiliation": [],
"authenticated-orcid": false,
"family": "Peto",
"given": "Leon",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-9759-0232",
"affiliation": [],
"authenticated-orcid": false,
"family": "Campbell",
"given": "Mark",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9826-6322",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wade",
"given": "Rachel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4050-6191",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pessoa-Amorim",
"given": "Guilherme",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0009-2808-8036",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tobert",
"given": "Vanessa",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4482-4418",
"affiliation": [],
"authenticated-orcid": false,
"family": "Staplin",
"given": "Natalie",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7792-9422",
"affiliation": [],
"authenticated-orcid": false,
"family": "Emberson",
"given": "Jonathan R",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0971-3026",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wallendszus",
"given": "Karl",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8474-4645",
"affiliation": [],
"authenticated-orcid": false,
"family": "Stevens",
"given": "William M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "King",
"given": "Andy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kurien",
"given": "Rijo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crichton",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brightling",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prudon",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Green",
"given": "Christopher A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thackoorcharan",
"given": "Sheri",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0202-608X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hine",
"given": "Paul",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6868-6633",
"affiliation": [],
"authenticated-orcid": false,
"family": "Felton",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stewart",
"given": "Richard",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0380-1116",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kunst",
"given": "Heinke",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6134-7855",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5258-793X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Baillie",
"given": "J Kenneth",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8962-5642",
"affiliation": [],
"authenticated-orcid": false,
"family": "Buch",
"given": "Maya H",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3410-7642",
"affiliation": [],
"authenticated-orcid": false,
"family": "Faust",
"given": "Saul N",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1096-188X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jaki",
"given": "Thomas",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6506-2689",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jeffery",
"given": "Katie",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5500-2247",
"affiliation": [],
"authenticated-orcid": false,
"family": "Juszczak",
"given": "Edmund",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1984-4575",
"affiliation": [],
"authenticated-orcid": false,
"family": "Knight",
"given": "Marian",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7694-3051",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lim",
"given": "Wei Shen",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0450-1606",
"affiliation": [],
"authenticated-orcid": false,
"family": "Montgomery",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mukherjee",
"given": "Aparna",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5523-511X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mumford",
"given": "Andrew",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8217-5602",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rowan",
"given": "Kathryn",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2858-2087",
"affiliation": [],
"authenticated-orcid": false,
"family": "Thwaites",
"given": "Guy",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0562-3963",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mafham",
"given": "Marion",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1179-0023",
"affiliation": [],
"authenticated-orcid": false,
"family": "Haynes",
"given": "Richard",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6646-827X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Landray",
"given": "Martin J",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
9,
3
]
],
"date-time": "2025-09-03T01:05:16Z",
"timestamp": 1756861516000
},
"deposited": {
"date-parts": [
[
2025,
9,
3
]
],
"date-time": "2025-09-03T01:05:16Z",
"timestamp": 1756861516000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2025,
9,
3
]
],
"date-time": "2025-09-03T01:40:24Z",
"timestamp": 1756863624215,
"version": "3.44.0"
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
9,
2
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
2
]
],
"date-time": "2025-09-02T00:00:00Z",
"timestamp": 1756771200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2025.08.29.25334732",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
9,
2
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2025,
9,
2
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2025.08.29.25334732"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Long-term follow-up of treatment comparisons in RECOVERY: a randomised, open-label, platform trial for patients hospitalised with COVID-19",
"type": "posted-content"
}
horby7