Targeting the Microbiome With KB109 in Outpatients with Mild to Moderate COVID-19 Reduced Medically Attended Acute Care Visits and Improved Symptom Duration in Patients With Comorbidities
et al., medRxiv, doi:10.1101/2021.03.26.21254422, NCT04414124, Mar 2021
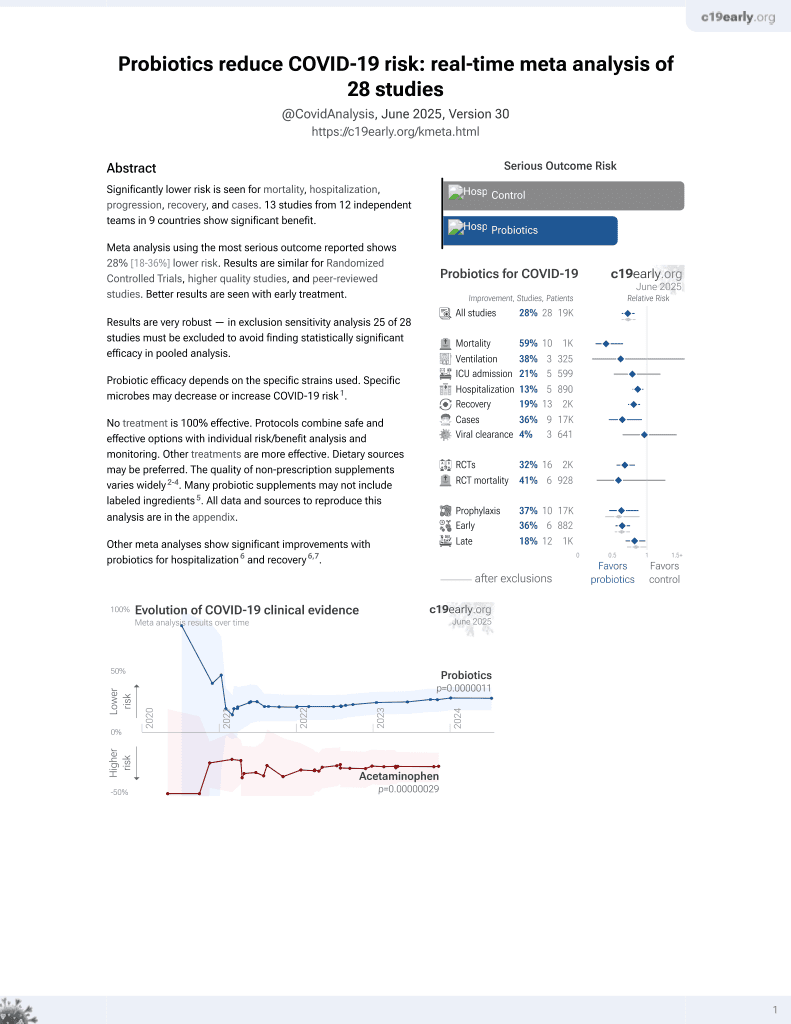
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 350 COVID+ outpatients in the USA, 174 treated with prebiotic KB109 (a microbiome metabolic therapy candidate), showing lower combined hospitalization, ER, and urgent care visits with treatment. NCT04414124 (history).
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Although the 67% lower mortality is not statistically significant, it is consistent with the significant 59% lower mortality [35‑74%] from meta-analysis of the 10 mortality results to date.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 66.5% lower, RR 0.33, p = 1.00, treatment 0 of 174 (0.0%), control 1 of 176 (0.6%), NNT 176, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), death two weeks after study withdrawal.
|
|
risk of hospitalization, 59.5% lower, RR 0.40, p = 0.45, treatment 2 of 174 (1.1%), control 5 of 176 (2.8%), NNT 59, including treatment period.
|
|
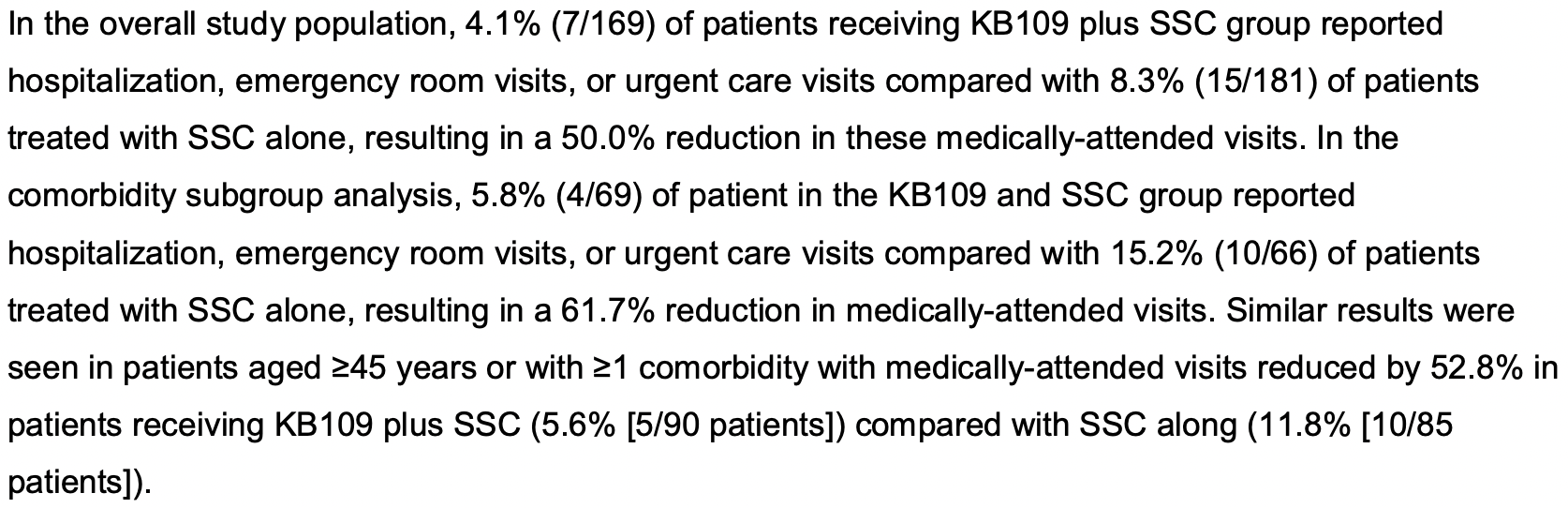
risk of hospitalization/ER/urgent care, 50.0% lower, RR 0.50, p = 0.13, treatment 7 of 169 (4.1%), control 15 of 181 (8.3%), NNT 24.
|
|
time to resolution of symptoms, 20.3% lower, relative time 0.80, p = 0.10, treatment 169, control 172, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Haran et al., 29 Mar 2021, Randomized Controlled Trial, USA, preprint, 6 authors, study period 2 July, 2020 - 23 December, 2020, trial NCT04414124 (history).
Targeting the Microbiome With KB109 in Outpatients with Mild to Moderate COVID-19 Reduced Medically Attended Acute Care Visits and Improved Symptom Duration in Patients With Comorbidities
doi:10.1101/2021.03.26.21254422
Methods Adult patients who tested positive for COVID-19 were randomized 1:1 to receive KB109 combined with SSC or SSC alone for 14 days and were then followed for an additional 21 days (35 days in total). Patients self-assessed their COVID-19-related symptoms (8 cardinal symptoms plus 5 additional symptoms) and self-reported comorbidities. The primary and secondary objectives were to evaluate the safety of KB109 plus SSC compared with that of SSC alone and to evaluate selected measures of health, respectively.
Results Between July 2, 2020 and December 23, 2020, 350 patients were randomized to receive KB109 and SSC (n=174) or SSC alone (n=176). Overall, the most common comorbidities reported were hypertension (18.0% [63/350 patients]) followed by chronic lung disease (8.6% 30/350 patients). KB109 was well tolerated with most treatment-emergent adverse events being mild to moderate in severity. The administration of KB109 plus SSC reduced medically-attended visits (ie, hospitalization, emergency room visits, or urgent care visits) by 50.0% in the overall population and by 61.7% in patients with ≥1 comorbidity; in patients aged ≥45 years or with ≥1 comorbidity, medically-attended visits were reduced by 52.8%, In the SSC group, patients reporting ≥1 comorbidity had a longer median time to resolution of symptoms than those who reported no comorbidities at baseline (13 overall symptoms: 30 vs 21 days, respectively; hazard ratio [HR]=1.163 [95% CI, 0.723-1.872]; 8 cardinal symptoms: 21 vs 15 days, respectively; HR=1.283 [95% CI, 0.809-2.035]). In patients reporting ≥1 comorbidity, median time to resolution of symptoms was shorter in the KB109 plus SSC group compared with the SSC alone group . (13 overall symptoms: 30 vs 21 days, respectively; HR=1.422 [95% CI, 0.898-2.250]; 8 cardinal symptoms: 17 vs 21 days, respectively; HR=1.574 [95% CI, 0.997-2.485]). In the KB109 plus SSC group, patients aged ≥45 years or with ≥1 comorbidity had a shorter median time to resolution of symptoms compared with SSC alone (overall 13 symptoms: 21 vs 31 days; HR=1.597 [95% CI, 1.064-2.398]).
Conclusions Results from our study show that KB109 is well tolerated among patients with mild to moderate COVID-19. Patients with ≥1 comorbidity had a longer duration of COVID-19 symptoms than those without comorbidities. Moreover, in patients reporting ≥1 comorbidity or aged ≥45 years (at-risk population), administration of KB109 plus SSC improved median time to resolution of COVID-19-related symptoms and reduced the rate of medically-attended visits compared with SSC alone.
Ethics Approval The authors ensure this study was conducted in full conformity with Regulations for the Protection of
Competing Interest Statement JPH has nothing to disclose. YZ, KK, NAP, and MAW are employees of and hold stock in Kaleido Biosciences, Inc. JFL is an employee of Kaleido Biosciences, Inc and has patents that are relevant to this work.
References
Ali, Zibert, Thomsen, Virtual clinical trials: perspectives in dermatology, Dermatology
Belkaid, Harrison, Homeostatic immunity and the microbiota, Immunity
Blueshield, Infographic: COVID-19 patients with high-risk conditions 3x more likely to need the ICU
Calo, Murray, Francis, Bermudez, Reaching the hispanic community about COVID-19 through existing chronic disease prevention programs, Prev Chronic Dis
Cdc Website, New variants of the virus that causes COVID-19
Chastain, Osae, Henao-Martínez, Franco-Paredes, Chastain et al., Racial disproportionality in Covid clinical trials, N Engl J Med
Chiu, Bazin, Truchetet, Schaeverbeke, Delhaes et al., Protective microbiota: from localized to long-reaching co-immunity, Front Immunol
Cohen, Hall, John, Rapoport, The early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic, Mayo Clin Proc
Dhar, Mohanty, Gut microbiota and Covid-19-possible link and implications, Virus Res
Gou, Fu, Yue, Gut microbiota may underlie the predisposition of healthy individuals to COVID-19, medRxiv, doi:10.1101/2020.04.22.20076091
Gu, Chen, Wu, Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza, Clin Infect Dis
Haak, Littmann, Chaubard, Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT, Blood
Hopkins, Global map
Huang, Huang, Wang, 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study, The Lancet
Ichinohe, Pang, Kumamoto, Microbiota regulates immune defense against respiratory tract influenza A virus infection, Proc Natl Acad Sci U S A
Keely, Talley, Hansbro, Pulmonary-intestinal cross-talk in mucosal inflammatory disease, Mucosal Immunol
Lechien, Chiesa-Estomba, Place, Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019, J Intern Med
Machhi, Herskovitz, Senan, The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections, J Neuroimmune Pharmacol
Meisner, Lawrence, Lee, Roed, Van et al., Development of a novel synthetic glycan to prevent bacterial infections and meliorate respiratory viral infections
Podewils, Burket, Mettenbrink, Disproportionate Incidence of COVID-19 Infection, Hospitalizations, and Deaths Among Persons Identifying as Hispanic or Latino -Denver, Colorado March-October 2020, MMWR Morb Mortal Wkly Rep
Sanyaolu, Okorie, Marinkovic, Comorbidity and its impact on patients with COVID-19, SN Compr Clin Med
Trompette, Gollwitzer, Pattaroni, Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism, Immunity
Venegas, De La Fuente, Landskron, Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases, Front Immunol
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
Wu, Chen, Cai, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med
Xie, Ma, Tang, Liu, Severe COVID-19: a review of recent progress with a look toward the future, Front Public Health
Yeoh, Zuo, Lui, Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut
DOI record:
{
"DOI": "10.1101/2021.03.26.21254422",
"URL": "http://dx.doi.org/10.1101/2021.03.26.21254422",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Introduction</jats:title><jats:p>In 2020, the world experienced the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also known as the coronavirus disease 2019 (COVID-19) pandemic. Mounting evidence indicates that the gut microbiome plays a role in host immune response to infections and, in turn, may have an impact on the disease trajectory of SARS-CoV2 infection. However, it remains to be established whether modulation of the microbiome can impact COVID-19–related symptomatology and patient outcomes. Therefore, we conducted a study designed to modulate the microbiome evaluating the safety and physiologic effects of KB109 combined with self-supportive care (SSC) vs SSC alone in non-hospitalized patients with mild to moderate COVID-19. KB109 is a novel synthetic glycan developed to increase the production of gut microbial metabolites that support immune system homeostasis through gut microbiome modulation. Our goal was to gain a better understanding of the safety of KB109, the natural course of COVID-19 symptomatology, and the possible role of the gut microbiome in patients with mild to moderate COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Adult patients who tested positive for COVID-19 were randomized 1:1 to receive KB109 combined with SSC or SSC alone for 14 days and were then followed for an additional 21 days (35 days in total). Patients self-assessed their COVID-19–related symptoms (8 cardinal symptoms plus 5 additional symptoms) and self-reported comorbidities. The primary and secondary objectives were to evaluate the safety of KB109 plus SSC compared with that of SSC alone and to evaluate selected measures of health, respectively.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Between July 2, 2020 and December 23, 2020, 350 patients were randomized to receive KB109 and SSC (n=174) or SSC alone (n=176). Overall, the most common comorbidities reported were hypertension (18.0% [63/350 patients]) followed by chronic lung disease (8.6% 30/350 patients). KB109 was well tolerated with most treatment-emergent adverse events being mild to moderate in severity. The administration of KB109 plus SSC reduced medically-attended visits (ie, hospitalization, emergency room visits, or urgent care visits) by 50.0% in the overall population and by 61.7% in patients with ≥1 comorbidity; in patients aged ≥45 years or with ≥1 comorbidity, medically-attended visits were reduced by 52.8%, In the SSC group, patients reporting ≥1 comorbidity had a longer median time to resolution of symptoms than those who reported no comorbidities at baseline (13 overall symptoms: 30 vs 21 days, respectively; hazard ratio [HR]=1.163 [95% CI, 0.723-1.872]; 8 cardinal symptoms: 21 vs 15 days, respectively; HR=1.283 [95% CI, 0.809-2.035]). In patients reporting ≥1 comorbidity, median time to resolution of symptoms was shorter in the KB109 plus SSC group compared with the SSC alone group (13 overall symptoms: 30 vs 21 days, respectively; HR=1.422 [95% CI, 0.898-2.250]; 8 cardinal symptoms: 17 vs 21 days, respectively; HR=1.574 [95% CI, 0.997-2.485]). In the KB109 plus SSC group, patients aged ≥45 years or with ≥1 comorbidity had a shorter median time to resolution of symptoms compared with SSC alone (overall 13 symptoms: 21 vs 31 days; HR=1.597 [95% CI, 1.064-2.398]).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Results from our study show that KB109 is well tolerated among patients with mild to moderate COVID-19. Patients with ≥1 comorbidity had a longer duration of COVID-19 symptoms than those without comorbidities. Moreover, in patients reporting ≥1 comorbidity or aged ≥45 years (at-risk population), administration of KB109 plus SSC improved median time to resolution of COVID-19–related symptoms and reduced the rate of medically-attended visits compared with SSC alone.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
3,
29
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7311-1121",
"affiliation": [],
"authenticated-orcid": false,
"family": "Haran",
"given": "John P.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knobil",
"given": "Katharine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palma",
"given": "Norma Alonzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lawrence",
"given": "Jonathan F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wingertzahn",
"given": "Mark A.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
29
]
],
"date-time": "2021-03-29T13:25:19Z",
"timestamp": 1617024319000
},
"deposited": {
"date-parts": [
[
2021,
3,
31
]
],
"date-time": "2021-03-31T09:15:33Z",
"timestamp": 1617182133000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T10:56:19Z",
"timestamp": 1640170579934
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
3,
29
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.03.26.21254422",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
3,
29
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
3,
29
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1007/s11481-020-09944-5",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.1"
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.2",
"unstructured": "Johns Hopkins. Global map. Updated February 9, 2021. Accessed March 19, 2021. https://coronavirus.jhu.edu/map.html."
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.3",
"unstructured": "CDC website. New variants of the virus that causes COVID-19. Updated February 2, 2021. Accessed February 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html."
},
{
"DOI": "10.1111/joim.13089",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.4"
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.5",
"unstructured": "CDC website. Symptoms of coronavirus. Updated December 22, 2020. Accessed February 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html."
},
{
"DOI": "10.3389/fpubh.2020.00189",
"article-title": "Severe COVID-19: a review of recent progress with a look toward the future",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "Front Public Health",
"key": "2021033102150738000_2021.03.26.21254422v1.6",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.04.010",
"article-title": "The early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic",
"doi-asserted-by": "crossref",
"first-page": "1124",
"issue": "6",
"journal-title": "Mayo Clin Proc",
"key": "2021033102150738000_2021.03.26.21254422v1.7",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)32656-8",
"article-title": "6-month consequences of COVID-19 in patients discharged from hospital: a cohort study",
"doi-asserted-by": "crossref",
"first-page": "220",
"issue": "10270",
"journal-title": "The Lancet",
"key": "2021033102150738000_2021.03.26.21254422v1.8",
"volume": "397",
"year": "2021"
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.9",
"unstructured": "CDC website. People with certain medical conditions. Updated February 3, 2021. Accessed February 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html"
},
{
"DOI": "10.1007/s42399-020-00363-4",
"doi-asserted-by": "crossref",
"key": "2021033102150738000_2021.03.26.21254422v1.10",
"unstructured": "Sanyaolu A , Okorie C , Marinkovic A , et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020:1–8."
},
{
"DOI": "10.1016/j.immuni.2017.04.008",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.11"
},
{
"DOI": "10.1182/blood-2018-01-828996",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.12"
},
{
"DOI": "10.1016/j.immuni.2018.04.022",
"article-title": "Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism",
"doi-asserted-by": "crossref",
"first-page": "992",
"issue": "5",
"journal-title": "Immunity",
"key": "2021033102150738000_2021.03.26.21254422v1.13",
"volume": "48",
"year": "2018"
},
{
"DOI": "10.1016/j.virusres.2020.198018",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.14"
},
{
"DOI": "10.3389/fimmu.2017.01678",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.15"
},
{
"DOI": "10.1038/mi.2011.55",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.16"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.17"
},
{
"DOI": "10.1093/cid/ciaa709",
"article-title": "Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza",
"doi-asserted-by": "crossref",
"first-page": "2669",
"issue": "10",
"journal-title": "Clin Infect Dis",
"key": "2021033102150738000_2021.03.26.21254422v1.18",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1019378108",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.19"
},
{
"DOI": "10.1101/2020.04.22.20076091",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.20"
},
{
"DOI": "10.3389/fimmu.2019.00277",
"article-title": "Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Front Immunol",
"key": "2021033102150738000_2021.03.26.21254422v1.21",
"volume": "10",
"year": "2019"
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.22",
"unstructured": "Meisner J , Lawrence J , Lee J , Roed M , van Hylckama Vlieg Jet . Development of a novel synthetic glycan to prevent bacterial infections and meliorate respiratory viral infections. Presented at: IDWeek Interactive Program. October 22–25, 2020."
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.23",
"unstructured": "European Medicines Agency. REGN-COV2 antibody combination (casirivimab/imdevimab) - COVID19 - Article-5(3) procedure: Conditions of use, conditions for distribution and patients targeted conditions for safety monitoring. First published February 26, 2021. Accessed March 24, 2021. https://www.ema.europa.eu/en/news/ema-issues-advice-use-regn-cov2-antibody-combination-casirivimab-imdevimab."
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.24",
"unstructured": "BlueCross BlueShield. Infographic: COVID-19 patients with high-risk conditions 3x more likely to need the ICU. Published February 9, 2021. Accessed March 26, 2021. https://www.bcbs.com/coronavirus-updates/stories/infographic-covid-19-patients-high-risk-conditions-3x-more-likely-need-the-icu."
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"article-title": "Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China",
"doi-asserted-by": "crossref",
"first-page": "934",
"issue": "7",
"journal-title": "JAMA Intern Med",
"key": "2021033102150738000_2021.03.26.21254422v1.25",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "publisher",
"key": "2021033102150738000_2021.03.26.21254422v1.26"
},
{
"key": "2021033102150738000_2021.03.26.21254422v1.27",
"unstructured": "US Department of Health & Human Services. Public Health Emergency. Bamlanivimab: update on COVID-19 variants and impact on bamlanivimab distribution. Published March 24, 2021. Accessed March 26, 2021. https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Bamlanivimab/Pages/default.aspx."
},
{
"DOI": "10.15585/mmwr.mm6948a3",
"article-title": "Disproportionate Incidence of COVID-19 Infection, Hospitalizations, and Deaths Among Persons Identifying as Hispanic or Latino - Denver, Colorado March-October 2020",
"doi-asserted-by": "crossref",
"first-page": "1812",
"issue": "48",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2021033102150738000_2021.03.26.21254422v1.28",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2021971",
"article-title": "Racial disproportionality in Covid clinical trials",
"doi-asserted-by": "crossref",
"first-page": "e59",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "2021033102150738000_2021.03.26.21254422v1.29",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.5888/pcd17.200165",
"doi-asserted-by": "crossref",
"key": "2021033102150738000_2021.03.26.21254422v1.30",
"unstructured": "Calo WA , Murray A , Francis E , Bermudez M , J. K. Reaching the hispanic community about COVID-19 through existing chronic disease prevention programs. Prev Chronic Dis. 17:200165. DOI: http://dx.doi.org/200110.205888/pcd200117.200165."
},
{
"DOI": "10.1159/000506418",
"article-title": "Virtual clinical trials: perspectives in dermatology",
"doi-asserted-by": "crossref",
"first-page": "375",
"issue": "4",
"journal-title": "Dermatology",
"key": "2021033102150738000_2021.03.26.21254422v1.31",
"volume": "236",
"year": "2020"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Targeting the Microbiome With KB109 in Outpatients with Mild to Moderate COVID-19 Reduced Medically Attended Acute Care Visits and Improved Symptom Duration in Patients With Comorbidities"
],
"type": "posted-content"
}