Viral glycoprotein-mediated entry and antibody-mediated immunity in HIV-1 and SARS-CoV-2 infection
et al., Frontiers in Immunology, doi:10.3389/fimmu.2025.1733684, Jan 2026
Review article examining class 1 viral fusion glycoproteins in HIV-1 and SARS-CoV-2, focusing on viral entry mechanisms and antibody-mediated neutralization strategies.
Grunst et al., 9 Jan 2026, peer-reviewed, 3 authors.
Contact: grunst@biochem.mpg.de, wenwei.li@yale.edu, walther.mothes@yale.edu.
Viral glycoprotein-mediated entry and antibody-mediated immunity in HIV-1 and SARS-CoV-2 infection
Frontiers in Immunology, doi:10.3389/fimmu.2025.1733684
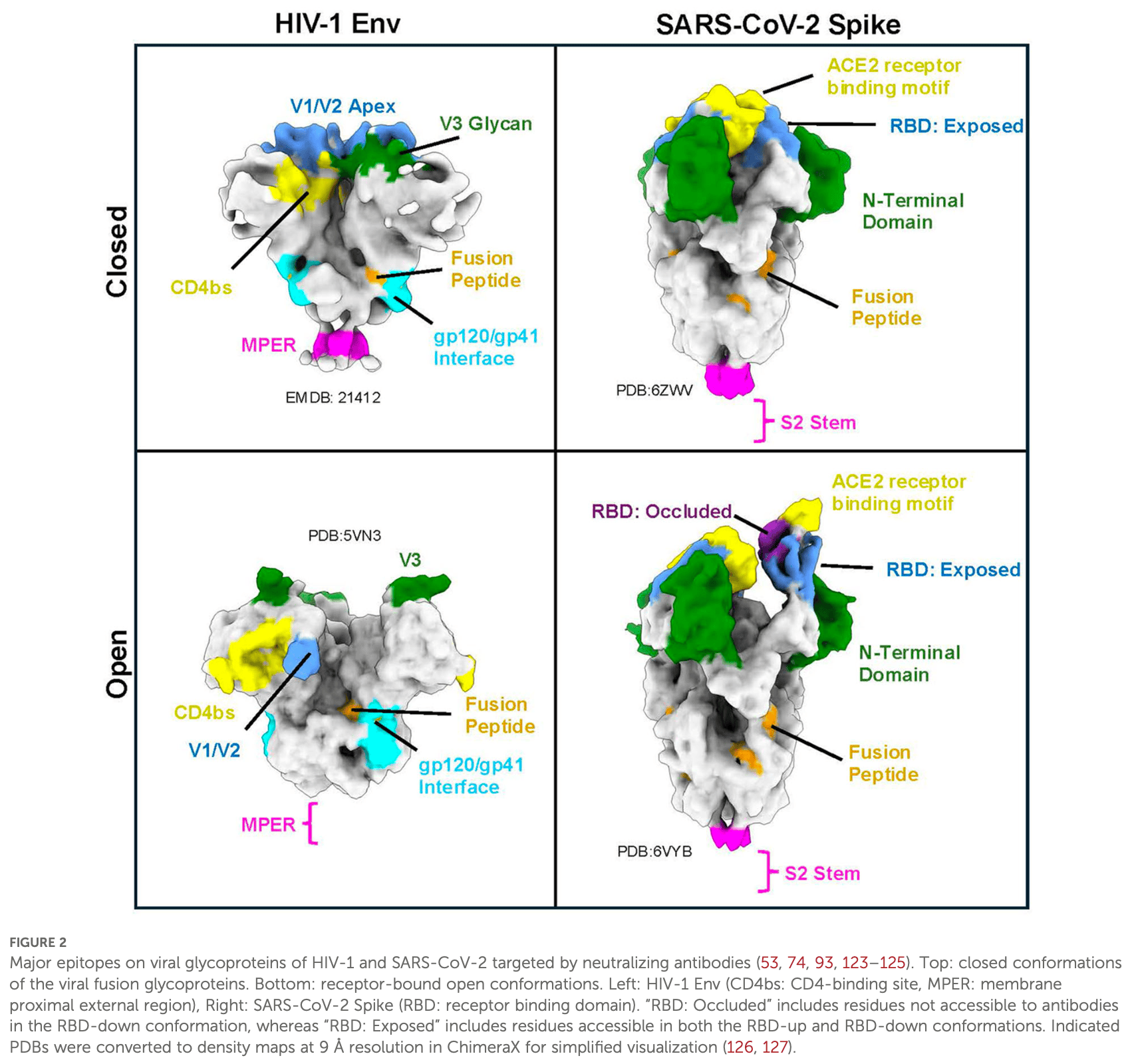
Enveloped viruses such as Human Immunodeficiency Virus (HIV-1) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) have caused some of the deadliest pandemics in human history. These viruses utilize Class 1 viral fusion glycoproteins to bind their host receptor and subsequently fuse the virus and host cell membranes to mediate entry. Viral fusion glycoproteins are prominent antigens on the surface of virions and are essential for the virus life cycle. Therefore, they are a primary target for the humoral immune system and the basis for the design of vaccines. Antibodies which target viral fusion glycoproteins can neutralize viral infectivity and activate the immune system in several distinct ways. In this review, we compare mechanisms of how class 1 viral fusion glycoproteins mediate viral entry and cover diverse ways in which antibodies targeting these glycoproteins can neutralize viruses and activate the immune system to clear virus-infected cells.
Conflict of interest The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a past co-authorship with the authors WL and WM.
Generative AI statement The author(s) declared that generative AI was not used in the creation of this manuscript. Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya, Tolbert, Gohain, Wu, Yu et al., Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection, J Virol, doi:10.1128/JVI.02194-14
Akil, Xu, Shen, Zhang, Unveiling the structural spectrum of SARS-CoV-2 fusion by in situ cryo-ET, Nat Commun, doi:10.1038/s41467-025-60406-z
Akimov, Molotkovsky, Kuzmin, Galimzyanov, Batishchev, Continuum models of membrane fusion: evolution of the theory, Int J Mol Sci, doi:10.3390/ijms21113875
Alam, Morelli, Dennison, Liao, Zhang et al., Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies, Proc Natl Acad Sci, doi:10.1126/science.1111781
Alsahafi, Bakouche, Kazemi, Richard, Ding et al., An asymmetric opening of HIV-1 envelope mediates antibody-dependent cellular cytotoxicity, Cell Host Microbe, doi:10.1016/j.chom.2019.03.002
Amanat, Thapa, Lei, Ahmed, Adelsberg et al., SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2, N Engl J Med, doi:10.1056/NEJMoa2035389
Anand, Grover, Tolbert, Prevost, Richard et al., Antibodyinduced internalization of HIV-1 env proteins limits surface expression of the closed conformation of env, J Virol, doi:10.1128/JVI.00293-19
Ananthaswamy, Fang, Alsalmi, Jain, Chen et al., A sequestered fusion peptide in the structure of an HIV-1 transmitted founder envelope trimer, Nat Commun, doi:10.1038/s41467-019-08825-7
Arias, Heyer, Bredow, Weisgrau, Moldt et al., Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.1321507111
Bangaru, Zhang, Gilchuk, Voss, Irving et al., A multifunctional human monoclonal neutralizing antibody that targets a unique conserved epitope on influenza HA, Nat Commun, doi:10.1038/s41467-018-04704-9
Barnes, Jette, Abernathy, Dam, Esswein et al., SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies, Nature, doi:10.1038/s41586-020-2852-1
Benton, Wrobel, Xu, Roustan, Martin et al., Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion, Nature, doi:10.1038/s41586-020-2772-0
Berger, Murphy, Farber, Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease, Annu Rev Immunol, doi:10.1146/annurev.immunol.17.1.657
Brady, Phelps, Macdonald, Lam, Nitido et al., Antibody-mediated prevention of vaginal HIV transmission is dictated by IgG subclass in humanized mice, Sci Transl Med, doi:10.1126/scitranslmed.abn9662
Brandenberg, Magnus, Rusert, Regoes, Trkola, Different infectivity of HIV-1 strains is linked to number of envelope trimers required for entry, PloS Pathog, doi:10.1371/journal.ppat.1004595
Bruhns, Iannascoli, England, Mancardi, Fernandez et al., Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses, Blood, doi:10.1182/blood-2008-09-179754
Bullough, Hughson, Skehel, Wiley, Structure of influenza haemagglutinin at the pH of membrane fusion, Nature, doi:10.1038/371037a0
Burton, Hessell, Keele, Klasse, Ketas et al., Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody, Proc Natl Acad Sci U.S.A, doi:10.1016/j.ebiom.2016.11.024
Burton, Mascola, Antibody responses to envelope glycoproteins in HIV-1 infection, Nat Immunol, doi:10.1038/ni.3158
Buzon, Natrajan, Schibli, Campelo, Kozlov et al., Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions, PloS Pathog, doi:10.1371/journal.ppat.1000880
Calder, Rosenthal, Cryomicroscopy provides structural snapshots of influenza virus membrane fusion, Nat Struct Mol Biol, doi:10.1038/nsmb.3271
Cao, Yisimayi, Jian, Song, Wang, 2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection, Nature, doi:10.1038/s41586-022-04980-y
Carabelli, Peacock, Thorne, Harvey, Hughes et al., SARS-CoV-2 variant biology: immune escape, transmission and fitness, Nat Rev Microbiol, doi:10.1038/s41579-022-00841-7
Carr, Kim, A spring-loaded mechanism for the conformational change of influenza hemagglutinin, Cell, doi:10.1016/0092-8674(93)90260-W
Caskey, Klein, Lorenzi, Seaman, West et al., Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117, Nature, doi:10.1038/nature14411
Chen, Zhang, Hwang, Bouton-Verville, Xia et al., Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10, J Immunol, doi:10.1016/j.cell.2024.04.033
Chen, Zhao, Zhou, Zhu, Jiang et al., Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses, Nat Rev Immunol, doi:10.1038/s41577-022-00784-3
Chernomordik, Kozlov, Mechanics of membrane fusion, Nat Struct Mol Biol, doi:10.1038/nsmb.1455
Chernomordik, Kozlov, Protein-lipid interplay in fusion and fission of biological membranes, Annu Rev Biochem, doi:10.1146/annurev.biochem.72.121801.161504
Chernomordik, Zimmerberg, Kozlov, Membranes of the world unite!, J Cell Biol, doi:10.1083/jcb.200607083
Chlanda, Mekhedov, Waters, Schwartz, Fischer et al., The hemifusion structure induced by influenza virus haemagglutinin is determined by physical properties of the target membranes, Nat Microbiol, doi:10.1038/nmicrobiol.2016.50
Chmielewski, Wilson, Pintilie, Zhao, Chen et al., Structural insights into the modulation of coronavirus spike tilting and infectivity by hinge glycans, Nat Commun, doi:10.1016/j.bpj.2020.10.036
Choe, Farzan, Sun, Sullivan, Rollins et al., The betachemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates, Cell, doi:10.1016/S0092-8674(00)81313-6
Chojnacki, Staudt, Glass, Bingen, Engelhardt et al., Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy, Science, doi:10.1126/science.1226359
Chojnacki, Waithe, Carravilla, Huarte, Galiani et al., Envelope glycoprotein mobility on HIV-1 particles depends on the virus maturation state, Nat Commun, doi:10.1038/s41467-017-00515-6
Chu, Crowley, Backes, Chang, Tay et al., Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies, PloS Pathog, doi:10.1371/journal.ppat.1008083
Cohen, Van Doremalen, Greaney, Andersen, Sharma et al., Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models, Science, doi:10.1016/j.cell.2024.07.052
Connor, Sheridan, Ceradini, Choe, Landau, Change in coreceptor use correlates with disease progression in HIV-1-infected individuals, J Exp Med, doi:10.1084/jem.185.4.621
Corey, Gilbert, Juraska, Montefiori, Morris et al., Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition, N Engl J Med, doi:10.1056/NEJMoa2031738
Dacon, Peng, Lin, Tucker, Lee et al., Rare, convergent antibodies targeting the stem helix broadly neutralize diverse betacoronaviruses, Cell Host Microbe, doi:10.1126/science.abj3321
Dacon, Tucker, Peng, Lee, Lin et al., Neutralization mechanism of a human antibody with pan-coronavirus reactivity including SARS-CoV-2, Nat Microbiol, doi:10.1038/s41564-022-01155-3
Dalgleish, Beverley, Clapham, Crawford, Greaves et al., The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus, Nature, doi:10.1038/312763a0
Dam, Fan, Yang, Bjorkman, Intermediate conformations of CD4bound HIV-1 Env heterotrimers, Nature, doi:10.1038/s41586-023-06639-8
Deng, Liu, Ellmeier, Choe, Unutmaz et al., Identification of a major co-receptor for primary isolates of HIV-1, Nature, doi:10.1038/381661a0
Dey, Pahari, Mukherjee, Munro, Das, Conformational dynamics of SARS-CoV-2 Omicron spike trimers during fusion activation at single molecule resolution, Structure, doi:10.1016/j.str.2024.09.008
Dey, Pahari, Shukla, Andrabi, Das et al., Single-molecule imaging prefusion intermediate conformations of MERS-CoV spike trimers in membrane during entry, Immunol Rev, doi:10.1038/s41590-018-0235-7
Diaz-Salinas, Jain, Durham, Munro, Single-molecule imaging reveals allosteric stimulation of SARS-CoV-2 spike receptor binding domain by host sialic acid, Sci Adv, doi:10.1126/sciadv.adk4920
Diaz-Salinas, Li, Ejemel, Yurkovetskiy, Luban et al., Conformational dynamics and allosteric modulation of the SARS-CoV-2 spike, Elife, doi:10.7554/eLife.75433
Ding, Tauzin, Pinto-Santini, Dasgupta, Yang et al., CD4-mimetics sensitize HIV-infected cells to ADCC mediated by plasma from persons with early-stage HIV-1 infection, J Virol, doi:10.1128/jvi.00858-25
Ding, Tolbert, Zhu, Lee, Marchitto et al., Cryo-EM structures of HIV-1 trimer bound to CD4-mimetics BNM-III-170 and M48U1 adopt a CD4-bound open conformation, Nat Commun, doi:10.1038/s41467-021-21816-x
Ding, Veillette, Coutu, Prevost, Scharf et al., A highly conserved residue of the HIV-1 gp120 inner domain is important for antibodydependent cellular cytotoxicity responses mediated by anti-cluster A antibodies, J Virol, doi:10.1128/JVI.02779-15
Dodero-Rojas, Onuchic, Whitford, Sterically confined rearrangements of SARS-CoV-2 Spike protein control cell invasion, Elife, doi:10.7554/eLife.70362
Dragic, Litwin, Allaway, Martin, Huang et al., HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5, Nature, doi:10.1038/381667a0
Dufloo, Planchais, Fremont, Lorin, Guivel-Benhassine et al., Broadly neutralizing anti-HIV-1 antibodies tether viral particles at the surface of infected cells, Nat Commun, doi:10.1038/s41467-022-28307-7
Egri, Wang, Diaz-Salinas, Luban, Dudkina et al., Detergent modulates the conformational equilibrium of SARS-CoV-2 Spike during cryo-EM structural determination, Nat Commun, doi:10.1038/s41467-023-38251-9
Fan, Cao, Kong, Zhang, Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein, Nat Commun, doi:10.1038/s41467-020-17371-6
Feng, Broder, Kennedy, Berger, HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor, Science, doi:10.1126/science.272.5263.872
Fernandez, Saunders, Duquerroy, Bolland, Arbabian et al., Structural basis of TMPRSS2 zymogen activation and recognition by the HKU1 seasonal coronavirus, Cell, doi:10.1016/j.cell.2024.06.007
Fritschi, Anang, Gong, Mohammadi, Richard et al., Indoline CD4-mimetic compounds mediate potent and broad HIV-1 inhibition and sensitization to antibody-dependent cellular cytotoxicity, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.2222073120
Fuhrmans, Marelli, Smirnova, Müller, Mechanics of membrane fusion/pore formation, Chem Phys Lipids, doi:10.1016/j.chemphyslip.2014.07.010
Garrett, Galloway, Wolf, Logue, Franko et al., Comprehensive characterization of the antibody responses to SARS-CoV-2 Spike protein finds additional vaccine-induced epitopes beyond those for mild infection, Elife, doi:10.1016/j.cell.2015.01.016
Ge, Wang, Ju, Zhang, Sun et al., Antibody neutralization of SARS-CoV-2 through ACE2 receptor mimicry, Nat Commun, doi:10.1038/s41467-020-20501-9
Goddard, Huang, Meng, Pettersen, Couch et al., UCSF ChimeraX: Meeting modern challenges in visualization and analysis, Nat Rev Immunol, doi:10.1038/s41577-023-00858-w
Gorman, Chuang, Lai, Shen, Boyington et al., Structure of super-potent antibody CAP256-VRC26.25 in complex with HIV-1 envelope reveals a combined mode of trimer-apex recognition, Cell Rep, doi:10.1016/j.celrep.2020.03.052
Gorman, Wang, Mason, Nazzari, Welles et al., Differences in the binding affinity of an HIV-1 V2 apex-specific antibody for the SIV, Nat Struct Mol Biol, doi:10.1128/mBio.01255-19
Griffith, Mccoy, To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1, Front Immunol, doi:10.3389/fimmu.2021.708227
Gristick, Boehmer, West, Jr, Schamber et al., Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site, Nat Struct Mol Biol, doi:10.1038/nsmb.3291
Gruell, Vanshylla, Tober-Lau, Hillus, Schommers et al., mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant, Nat Med, doi:10.21203/rs.3.rs-1168453/v1
Grunst, Gil, Grandea, Snow, Andrabi et al., Potent antibody-dependent cellular cytotoxicity of a V2-specific antibody is not sufficient for protection of macaques against SIV challenge, PloS Pathog, doi:10.1126/science.abh2315
Grunst, Ladd, Clark, Gil, Klenchin et al., Antibody-dependent cellular cytotoxicity, infected cell binding and neutralization by antibodies to the SIV envelope glycoprotein, PloS Pathog, doi:10.1128/JVI.00347-16
Grunst, Qin, Dodero-Rojas, Ding, Prevost et al., Structure and inhibition of SARS-CoV-2 spike refolding in membranes, Science, doi:10.1126/science.adn5658
Guenthoer, Garrett, Lilly, Depierreux, Ruiz et al., Convergent antibody responses to the SARS-CoV-2 spike protein in convalescent and vaccinated individuals, PloS Pathog, doi:10.1016/j.str.2024.08.022
Gunst, Gohil, Li, Bosch, White et al., Time to HIV viral rebound and frequency of post-treatment control after analytical interruption of antiretroviral therapy: an individual data-based meta-analysis of 24 prospective studies, Nat Commun, doi:10.1038/s41467-025-56116-1
Hachmann, Miller, Collier, Ventura, Yu et al., Neutralization escape by SARS-coV-2 omicron subvariants BA
Harrison, Viral membrane fusion, Nat Struct Mol Biol, doi:10.1038/nsmb.1456
Haynes, Gilbert, Mcelrath, Zolla-Pazner, Tomaras et al., Immune-correlates analysis of an HIV-1 vaccine efficacy trial, N Engl J Med, doi:10.1056/NEJMoa1113425
Hessell, Hangartner, Hunter, Havenith, Beurskens et al., Fc receptor but not complement binding is important in antibody protection against HIV, Nature, doi:10.1038/nature06106
Hessell, Poignard, Hunter, Hangartner, Tehrani et al., Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques, Nat Med, doi:10.1038/nm.1974
Hoffmann, Kleine-Weber, Pohlmann, A multibasic cleavage site in the spike protein of SARS-coV-2 is essential for infection of human lung cells, Mol Cell, doi:10.1016/j.molcel.2020.04.022
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hofmann, Pyrc, Van Der Hoek, Geier, Berkhout et al., Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.0409465102
Huang, Chen, Mohapatra, Nguyen, Schimanski et al., Structural basis for a conserved neutralization epitope on the receptor-binding domain of SARS-CoV-2, Nat Commun, doi:10.1038/s41467-023-35949-8
Huang, Kang, Ishida, Zhou, Griesman et al., Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth, Immunity, doi:10.1016/j.immuni.2016.10.027
Huang, Ofek, Laub, Louder, Rose et al., Broad and potent neutralization of HIV-1 by a gp41-specific human antibody, Nature, doi:10.1038/nature11544
Hurlburt, Homad, Sinha, Jennewein, Maccamy et al., Isolation and characterization of cross-neutralizing coronavirus antibodies from COVID-19+ subjects, Commun Biol, doi:10.1038/s41467-023-43638-9
Iliopoulou, Nolan, Alvarez, Watanabe, Coomer et al., A dynamic three-step mechanism drives the HIV-1 pre-fusion reaction, Nat Struct Mol Biol, doi:10.1038/s41594-018-0113-x
Ivan, Sun, Subbaraman, Friedrich, Trkola et al., CD4 occupancy triggers sequential pre-fusion conformational states of the HIV-1 envelope trimer with relevance for broadly neutralizing antibody activity, Nat Struct Mol Biol, doi:10.1038/nsmb0510-543
Jaumouille, Farkash, Jaqaman, Das, Lowell et al., Actin cytoskeleton reorganization by Syk regulates Fcgamma receptor responsiveness by increasing its lateral mobility and clustering, Front Immunol, doi:10.1186/s12915-020-00819-y
Kaplonek, Fischinger, Cizmeci, Bartsch, Kang et al., mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern, Immunity, doi:10.1016/j.immuni.2022.01.001
Katte, Xu, Han, Hong, Lu, Inter-protomer opening cooperativity of envelope trimers positively correlates with HIV-1 entry stoichiometry, mBio, doi:10.1128/mbio.02754-24
Ke, Oton, Qu, Cortese, Zila et al., Structures and distributions of SARS-CoV-2 spike proteins on intact virions, Nature, doi:10.1101/2020.06.27.174979
Kephart, Hom, Lee, Visualizing intermediate stages of viral membrane fusion by cryo-electron tomography, Trends Biochem Sci, doi:10.1016/j.tibs.2024.06.012
Klatzmann, Champagne, Chamaret, Gruest, Guetard et al., T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV, Nature, doi:10.1038/312767a0
Klein, Bjorkman, Doores, Walker, Khayat et al., A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield, PloS Pathog, doi:10.1126/science.1213256
Klein, Gnanapragasam, Galimidi, Foglesong, West et al., Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10, Proc Natl Acad Sci U.S.A, doi:10.1016/j.chom.2023.06.006
Koch, Uckeley, Doldan, Stanifer, Boulant et al., TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells, EMBO J, doi:10.15252/embj.2021107821
Kumar, Sarkar, Pugach, Sanders, Moore et al., Capturing the inherent structural dynamics of the HIV-1 envelope glycoprotein fusion peptide, Nat Commun, doi:10.1038/s41467-019-08738-5
Kuzmin, Zimmerberg, Chizmadzhev, Cohen, A quantitative model for membrane fusion based on low-energy intermediates, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.121191898
Ladinsky, Gnanapragasam, Yang, West, Kay et al., Electron tomography visualization of HIV-1 fusion with target cells using fusion inhibitors to trap the pre-hairpin intermediate, Elife, doi:10.7554/eLife.58411
Ladinsky, Zhu, Ullah, Uchil, Kumar et al., Electron tomography visualization of HIV-1 virions trapped by fusion inhibitors to host cells in infected tissues, J Virol, doi:10.1128/jvi.01432-24
Layne, Merges, Dembo, Spouge, Conley et al., Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus, Virology, doi:10.1016/0042-6822(92)90593-E
Lee, Andrabi, Su, Yasmeen, Julien et al., A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic beta-Hairpin Structure, Immunity, doi:10.1016/j.immuni.2017.03.017
Lee, Ozorowski, Ward, Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer, Science, doi:10.1126/science.aad2450
Lee, Selva, Davis, Wines, Reynaldi et al., The Fc-effector function of COVID-19 convalescent plasma contributes to SARS-CoV-2 treatment efficacy in mice, Cell Rep Med, doi:10.1016/j.xcrm.2022.100893
Leikina, Chernomordik, Reversible merger of membranes at the early stage of influenza hemagglutinin-mediated fusion, Mol Biol Cell, doi:10.1091/mbc.11.7.2359
Leonhardt, Purdy, Grover, Yang, Poulos et al., Antiviral HIV-1 SERINC restriction factors disrupt virus membrane asymmetry, Nat Commun, doi:10.1038/s41467-023-39262-2
Li, Chen, Prévost, Ullah, Lu et al., Mapping neutralizing and immunodominant sites on the SARS-coV-2 spike receptor-binding domain by structure-guided high-resolution serology, Cell Rep, doi:10.1016/j.cell.2020.09.037
Li, Dell, Walker, Wu, Guenaga et al., Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01, J Virol, doi:10.1128/JVI.00754-11
Li, Hulswit, Widjaja, Raj, Mcbride et al., Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein, Proc Natl Acad Sci, doi:10.1073/pnas.1712592114
Li, Lai, Zhang, Jiang, Tian et al., Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients, Cell Mol Immunol, doi:10.1038/s41423-020-00523-5
Li, Li, Lu, Jjr, Chao et al., Subnanometer structures of HIV-1 envelope trimers on aldrithiol-2-inactivated virus particles, Nat Struct Mol Biol, doi:10.1002/pro.3943
Li, Moore, Vasilieva, Sui, Wong et al., Angiotensinconverting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Li, Qin, Nand, Grunst, Grover et al., HIV-1 Env trimers asymmetrically engage CD4 receptors in membranes, Nature, doi:10.1038/s41586-023-06762-6
Li, Tomlinson, Wong, Zhou, Desforges et al., The human coronavirus HCoV-229E S-protein structure and receptor binding, Elife, doi:10.7554/eLife.51230
Liu, Casner, Guo, Wang, Iketani et al., Antibodies targeting a quaternary site on SARS-CoV-2 spike glycoprotein prevent viral receptor engagement by conformational locking, Immunity, doi:10.1016/j.immuni.2023.09.003
Liu, Wang, Nair, Yu, Rapp et al., Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike, Nature, doi:10.1038/s41586-020-2571-7
Liu, Yu, Jian, Yang, Song et al., Enhanced immune evasion of SARS-CoV-2 variants KP.3.1.1 and XEC through N-terminal domain mutations, Lancet Infect Dis, doi:10.1101/2024.10.23.619754
Low, Jerak, Tortorici, Mccallum, Pinto et al., ACE2binding exposes the SARS-CoV-2 fusion peptide to broadly neutralizing coronavirus antibodies, Science, doi:10.1126/science.abq2679
Lu, Hu, Wang, Qi, Gao et al., Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26, Nature, doi:10.1038/nature12328
Lu, Ma, Castillo-Menendez, Gorman, Alsahafi et al., Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET, Nature, doi:10.1038/s41586-019-1101-y
Lu, Uchil, Li, Zheng, Terry et al., Real-time conformational dynamics of SARS-coV-2 spikes on virus particles, Cell Host Microbe, doi:10.1016/j.chom.2020.11.001
Mackin, Desai, Whitener, Karl, Liu et al., Fc-gammaR-dependent antibody effector functions are required for vaccine-mediated protection against antigen-shifted variants of SARS-CoV-2, Nat Microbiol, doi:10.1038/s41564-023-01359-1
Madani, Princiotto, Mach, Ding, Prevost et al., A CD4mimetic compound enhances vaccine efficacy against stringent immunodeficiency virus challenge, Nat Commun, doi:10.1038/s41467-018-04758-9
Maddon, Dalgleish, Mcdougal, Clapham, Weiss et al., The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain, Cell, doi:10.1016/0092-8674(86)90590-8
Marcink, Kicmal, Armbruster, Zhang, Zipursky et al., Intermediates in SARS-CoV-2 spike-mediated cell entry, Sci Adv, doi:10.1126/sciadv.abo3153
Martinez, Diemert, Braibant, Potard, Charuel et al., Anticardiolipin antibodies in HIV infection are independently associated with antibodies to the membrane proximal external region of gp41 and with cellassociated HIV DNA and immune activation, Clin Infect Dis, doi:10.1086/595013
Mascola, Montefiori, HIV-1: nature's master of disguise, Nat Med, doi:10.1038/nm0403-393
Mazor, Yang, Borrok, Ayriss, Aherne et al., Enhancement of immune effector functions by modulating igG's intrinsic affinity for target antigen, N Engl J Med, doi:10.1056/NEJMoa0908492
Mccallum, Marco, Lempp, Tortorici, Pinto et al., Neutralizing and protective human monoclonal antibodies recognizing the Nterminal domain of the SARS-CoV-2 spike protein, Cell, doi:10.1016/j.cell.2021.03.029
Mccallum, Park, Stewart, Sprouse, Addetia et al., Human coronavirus HKU1 recognition of the TMPRSS2 host receptor, Cell, doi:10.1016/j.cell.2024.06.006
Mccormick, Jacobs, Mellors, The emerging plasticity of SARS-CoV-2, Science, doi:10.1126/science.abg4493
Mcdougal, Kennedy, Sligh, Cort, Mawle et al., Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule, Science, doi:10.1126/science.3001934
Melikyan, Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm, Retrovirology, doi:10.1186/1742-4690-5-111
Moldt, Shibata-Koyama, Rakasz, Schultz, Kanda et al., A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques, J Virol, doi:10.1128/JVI.00491-12
Munro, Gorman, Ma, Zhou, Arthos et al., Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions, Science, doi:10.1126/science.1254426
Nan, Li, Zhang, Wang, Lv et al., Exploring distinct modes of inter-spike cross-linking for enhanced neutralization by SARS-CoV-2 antibodies, Nat Commun, doi:10.1038/s41467-024-54746-5
Ng, Faulkner, Cornish, Rosa, Harvey et al., Preexisting and de novo humoral immunity to SARS-CoV-2 in humans, Science, doi:10.1126/science.abe1107
Nguyen, Mccord, Bui, Bouwman, Kitova et al., Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2, Nat Chem Biol, doi:10.1038/s41589-021-00924-1
Nimmerjahn, Ravetch, Saunders, Conceptual approaches to modulating antibody effector functions and circulation half-life, Nat Rev Immunol, doi:10.3389/fimmu.2019.01296
Ofek, Tang, Sambor, Katinger, Mascola et al., Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope, J Virol, doi:10.1128/JVI.78.19.10724-10737.2004
Olukitibi, Ao, Warner, Unat, Kobasa et al., Significance of conserved regions in coronavirus spike protein for developing a novel vaccine against SARS-coV-2 infection, Vaccines, doi:10.3390/vaccines11030545
Orlandi, Deredge, Ray, Gohain, Tolbert et al., Antigeninduced allosteric changes in a human igG1 fc increase low-affinity fcgamma receptor binding, Structure, doi:10.1016/j.str.2020.03.001
Ou, Liu, Lei, Li, Mi et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat Commun, doi:10.1038/s41467-020-15562-9
Ozorowski, Pallesen, De Val, Lyumkis, Cottrell et al., Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike, Nature, doi:10.1038/nature23010
Pancera, Zhou, Druz, Georgiev, Soto et al., Structure and immune recognition of trimeric pre-fusion HIV-1 Env, Nature, doi:10.1038/nature13808
Park, Li, Barlan, Fehr, Perlman et al., Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism, Proc Natl Acad Sci U.S.A, doi:10.1016/j.virol.2017.12.015
Park, Walls, Wang, Sauer, Li et al., Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors, Nat Struct Mol Biol, doi:10.1038/s41594-019-0334-7
Parsons, Lee, Kristensen, Amarasena, Khoury et al., Fc-dependent functions are redundant to efficacy of anti-HIV antibody PGT121 in macaques, J Clin Invest, doi:10.1172/JCI122466
Pierre, Adams, Higgins, Anasti, Goodman et al., Nonneutralizing SARS-CoV-2 N-terminal domain antibodies protect mice against severe disease using Fc-mediated effector functions, PloS Pathog, doi:10.1371/journal.ppat.1011569
Polack, Thomas, Kitchin, Absalon, Gurtman et al., Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Powell, Totrov, Itri, Liu, Fox et al., Plasticity and epitope exposure of the HIV-1 envelope trimer, J Virol, doi:10.1128/JVI.00410-17
Prasad, Leaman, Lovendahl, Croft, Benhaim et al., Cryo-ET of Env on intact HIV virions reveals structural variation and positioning on the Gag lattice, Cell, doi:10.1016/j.cell.2022.01.013
Prevost, Anand, Rajashekar, Zhu, Richard et al., HIV-1 Vpu restricts Fc-mediated effector functions in vivo, Cell Rep, doi:10.1016/j.celrep.2022.111624
Prevost, Medjahed, Vezina, Chen, Hahn et al., HIV-1 envelope glycoproteins proteolytic cleavage protects infected cells from ADCC mediated by plasma from infected individuals, Viruses, doi:10.3390/v13112236
Pronker, Creutznacher, Drulyte, Hulswit, Li et al., Sialoglycan binding triggers spike opening in a human coronavirus, Nature, doi:10.1038/s41586-023-06599-z
Qin, Kufareva, Holden, Wang, Zheng et al., Structural biology. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine, Science, doi:10.1126/science.1261064
Rajashekar, Richard, Beloor, Prevost, Anand et al., Modulating HIV-1 envelope glycoprotein conformation to decrease the HIV-1 reservoir, Cell Host Microbe, doi:10.1016/j.chom.2021.04.014
Rantalainen, Berndsen, Antanasijevic, Schijner, Zhang et al., HIV-1 envelope and MPER antibody structures in lipid assemblies, Cell Rep, doi:10.1016/j.celrep.2020.107583
Ren, Korom, Ward, Truong, Chan et al., Relationships between neutralization, binding, and ADCC of broadly neutralizing antibodies against reservoir HIV, J Virol, doi:10.1128/JVI.01808-20
Richard, Grunst, Niu, Diaz-Salinas, Zhu et al., The asymmetric opening of HIV-1 Env by a potent CD4 mimetic enables anti-coreceptor binding site antibodies to mediate ADCC, Nat Commun, doi:10.1038/s41467-025-65866-x
Richard, Prevost, Baxter, Bredow, Ding et al., Uninfected bystander cells impact the measurement of HIV-specific antibodydependent cellular cytotoxicity responses, mBio, doi:10.1128/mBio.00358-18
Richard, Sannier, Zhu, Prevost, Marchitto et al., CD4 downregulation precedes Env expression and protects HIV-1-infected cells from ADCC mediated by non-neutralizing antibodies, mBio, doi:10.1128/mbio.01827-24
Richard, Veillette, Ding, Zoubchenok, Alsahafi et al., Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity, EBioMedicine, doi:10.1016/j.ebiom.2015.12.004
Richardson, Manamela, Motsoeneng, Kaldine, Ayres et al., SARS-CoV-2 Beta and Delta variants trigger Fc effector function with increased cross-reactivity, Cell Rep Med, doi:10.1016/j.xcrm.2022.100510
Rosenblum, Wallace, Godfrey, Roper, Hall et al., Interim recommendations from the advisory committee on immunization practices for the use of bivalent booster doses of COVID-19 vaccines -United States, october 2022, MMWR Morb Mort Wkly Rep, doi:10.15585/mmwr.mm7145a2
Rychert, Strick, Bazner, Robinson, Rosenberg, Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines, AIDS Res Hum Retroviruses, doi:10.1089/aid.2009.0290
Santosuosso, Righi, Lindstrom, Leblanc, Poznansky, HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection, J Infect Dis, doi:10.1086/605695
Santra, Tomaras, Warrier, Nicely, Liao et al., Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques, Nat Rev Immunol, doi:10.1038/s41577-021-00649-1
Saunders, Fernandez, Planchais, Michel, Rajah et al., TMPRSS2 is a functional receptor for human coronavirus HKU1, Nature, doi:10.1038/s41586-023-06761-7
Scheid, Horwitz, Bar-On, Kreider, Lu et al., HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption, Nature, doi:10.1038/nature18929
Scheid, Mouquet, Ueberheide, Diskin, Klein et al., Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding, Science, doi:10.1126/science.1207227
Schibli, Weissenhorn, Class I and class II viral fusion protein structures reveal similar principles in membrane fusion, Mol Membr Biol, doi:10.1080/09687860400017784
Schlothauer, Herter, Koller, Grau-Richards, Steinhart et al., Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions, Protein Eng Des Sel, doi:10.1093/protein/gzw040
Shah, Canziani, Carter, Chaiken, The case for S2: the potential benefits of the S2 subunit of the SARS-coV-2 spike protein as an immunogen in fighting the COVID-19 pandemic, Front Immunol, doi:10.3389/fimmu.2021.637651
Shaik, Peng, Lu, Rits-Volloch, Xu et al., Structural basis of coreceptor recognition by HIV-1 envelope spike, Nature, doi:10.1038/s41586-018-0804-9
Shang, Wan, Luo, Ye, Geng et al., Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein, Proc Natl Acad Sci U.S.A, doi:10.1038/s41564-021-00908-w
Shi, Cai, Zhu, Peng, Voyer et al., Cryo-EM structure of SARS-CoV-2 postfusion spike in membrane, Nature, doi:10.1038/s41586-023-06273-4
Shi, Wang, Zhou, Sastry, Yang et al., Vaccine-elicited murine antibody WS6 neutralizes diverse beta-coronaviruses by recognizing a helical stem supersite of vulnerability, Structure, doi:10.1016/j.celrep.2021.109929
Shrock, Fujimura, Kula, Timms, Lee et al., Linear epitope landscape of the SARS-CoV-2 Spike protein constructed from 1,051 COVID-19 patients, Cell Rep, doi:10.1016/j.celrep.2021.108915
Singh, Mukherji, Basak, Hoffmann, Das et al., Dynamic Ca(2+) sensitivity stimulates the evolved SARS-CoV-2 spike strain-mediated membrane fusion for enhanced entry, Cell Rep, doi:10.1128/mbio.03227-21
Song, He, Callaghan, Anzanello, Huang et al., Crossreactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection, Nat Commun, doi:10.1038/s41467-021-23074-3
Soto, Lemmin, Chuang, Druz, Kong, Trimeric HIV-1-env structures define glycan shields from clades A, B and G. Cell, doi:10.1016/j.cell.2016.04.010
Stacey, Hrebik, Nand, Shetty, Qu et al., The conserved HIV-1 spacer peptide 2 triggers matrix lattice maturation, Nature, doi:10.1038/s41586-025-08624-9
Tauzin, Nayrac, Benlarbi, Gong, Gasser et al., A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses, Cell Host Microbe, doi:10.1016/j.chom.2021.06.001
Thakur, Katte, Xu, Janowska, Sammour et al., Conformational trajectory of the HIV-1 fusion peptide during CD4-induced envelope opening, Nat Commun, doi:10.1038/s41467-025-59721-2
Thompson, Natarajan, Irving, Rowley, Griggs et al., Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance -VISION network, 10 states, august 2021-january 2022, Morb Mortality Wkly Rep, doi:10.21203/rs.3.rs-1175516/v1
Tortorici, Walls, Joshi, Park, Eguia et al., Structure, receptor recognition, and antigenicity of the human coronavirus CCoV-HuPn-2018 spike glycoprotein, Cell, doi:10.1016/j.cell.2022.05.019
Tortorici, Walls, Lang, Wang, Li et al., Structural basis for human coronavirus attachment to sialic acid receptors, Nat Struct Mol Biol, doi:10.1038/s41594-019-0233-y
Turonova, Sikora, Schurmann, Hagen, Welsch et al., In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges, Science, doi:10.1016/j.cell.2020.09.018
Ullah, Prevost, Ladinsky, Stone, Lu et al., Live imaging of SARS-CoV-2 infection in mice reveals that neutralizing antibodies require Fc function for optimal efficacy, Immunity, doi:10.1016/j.immuni.2021.08.015
Valades-Alcaraz, Reinosa, Holguin, HIV transmembrane glycoprotein conserved domains and genetic markers across HIV-1 and HIV-2 variants, Front Microbiol, doi:10.3389/fmicb.2022.855232
Veillette, Coutu, Richard, Batraville, Dagher et al., The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibodydependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals, J Virol, doi:10.1128/JVI.02868-14
Verkoczy, Diaz, Holl, Ouyang, Bouton-Verville et al., Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance, Proc Natl Acad Sci U.S.A, doi:10.4049/jimmunol.1301285
Von Bredow, Arias, Heyer, Gardner, Farzan et al., Envelope glycoprotein internalization protects human and simian immunodeficiency virus-infected cells from antibody-dependent cell-mediated cytotoxicity, J Virol, doi:10.1128/JVI.01911-15
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, function, and antigenicity of the SARS-coV-2 spike glycoprotein, Cell, doi:10.1016/j.cell.2020.02.058
Wang, Cheng, Santo, Shen, Bylund et al., Potent and broad HIV-1 neutralization in fusion peptide-primed SHIV-infected macaques, Science, doi:10.1038/s41598-020-59711-y
Wang, Cohen, Galimidi, Gristick, Jensen et al., Cryo-EM structure of a CD4-bound open HIV-1 envelope trimer reveals structural rearrangements of the gp120 V1V2 loop, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.1615939113
Wang, Liu, Zhang, Zhao, Lu et al., TMPRSS2 and glycan receptors synergistically facilitate coronavirus entry, Cell, doi:10.1016/j.cell.2024.06.016
Wang, Zhan, Liu, Wang, Zhang et al., A broadly neutralizing antibody against SARS-CoV-2 Omicron variant infection exhibiting a novel trimer dimer conformation in spike protein binding, Cell Res, doi:10.1038/s41422-022-00684-0
Weitzenfeld, Bournazos, Ravetch, Antibodies targeting sialyl Lewis A mediate tumor clearance through distinct effector pathways, J Clin Invest, doi:10.1172/JCI128437
White, Delos, Brecher, Schornberg, Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme, Crit Rev Biochem Mol Biol, doi:10.1080/10409230802058320
Wilder-Smith, COVID-19 in comparison with other emerging viral diseases: risk of geographic spread via travel, Trop Dis Trav Med Vaccines, doi:10.1186/s40794-020-00129-9
Williams, Ofek, Schatzle, Mcdaniel, Lu et al., Potent and broad HIV-neutralizing antibodies in memory B cells and plasma, Sci Immunol, doi:10.1126/sciimmunol.aal2200
Winkler, Gilchuk, Yu, Bailey, Chen et al., Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection, Cell, doi:10.1016/j.cell.2021.02.026
Wong, Tomlinson, Zhou, Satkunarajah, Chen et al., Receptor-binding loops in alphacoronavirus adaptation and evolution, Nat Commun, doi:10.1038/s41467-017-01706-x
Wong, Zhou, Rini, Wa, Liu et al., Crystal structure of CD26/ dipeptidyl-peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface, J Biol Chem, doi:10.1074/jbc.M405001200
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Wu, Yang, Li, Hogerkorp, Schief et al., Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1, Science, doi:10.1126/science.1187659
Wyatt, Sodroski, The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens, Science, doi:10.1126/science.280.5371.1884
Xiang, Xu, Mcgovern, Ranzenigo, Huang et al., Functional interactions of common allotypes of rhesus macaque fcgR2A and fcgR3A with human and macaque igG subclasses, Immunol Cell Biol, doi:10.4049/jimmunol.2000501
Xing, Liu, Wang, Liu, Xu et al., Early fusion intermediate of ACE2-using coronavirus spike acting as an antiviral target, Cell, doi:10.1016/j.cell.2025.01.012
Xu, Acharya, Kong, Cheng, Chuang et al., Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1, Nat Med, doi:10.1038/s41591-018-0042-6
Xu, Alegre, Varga, Rothermel, Collins et al., In vitro characterization of five humanized OKT3 effector function variant antibodies, Cell Immunol, doi:10.1006/cimm.2000.1617
Yan, Wang, Ju, Yu, Zhang et al., Structural basis for bivalent binding and inhibition of SARS-CoV-2 infection by human potent neutralizing antibodies, Cell Res, doi:10.1038/s41422-021-00487-9
Yang, Hiotis, Wang, Chen, Wang et al., Dynamic HIV-1 spike motion creates vulnerability for its membrane-bound tripod to antibody attack, Nat Commun, doi:10.1038/s41467-022-34008-y
Yang, Kelkar, Manicassamy, Neelamegham, Conserved role of spike S2 domain N-glycosylation across betacoronaviruses, NPJ Viruses, doi:10.1038/s44298-024-00085-7
Yang, Wang, Liu, Gristick, Bjorkman, Asymmetric opening of HIV-1 Env bound to CD4 and a coreceptor-mimicking antibody, Nat Struct Mol Biol, doi:10.1038/s41594-019-0344-5
Ye, Liu, Li, Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain, Nat Commun, doi:10.1038/s41467-022-28882-9
Yeager, Ashmun, Williams, Cardellichio, Shapiro et al., Human aminopeptidase N is a receptor for human coronavirus 229, E. Nature, doi:10.1038/357420a0
Yu, Wang, Zhang, Li, Lu et al., Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients, Signal Transduct Target Ther, doi:10.1038/s41392-021-00759-1
Yuan, Cao, Zhang, Ma, Qi et al., Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains, Nat Commun, doi:10.1038/ncomms15092
Yuan, Cottrell, Ozorowski, Van Gils, Kumar et al., Antibodies targeting the fusion peptide on the HIV envelope provide protection to rhesus macaques against mucosal SHIV challenge, Emerg Microbes Infect, doi:10.1080/22221751.2023.2220582
Yuan, Wu, Zhu, Lee, So et al., A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV, Science, doi:10.1126/science.abb7269
Zhang, Irimia, He, Landais, Rantalainen et al., An MPER antibody neutralizes HIV-1 using germline features shared among donors, Nat Commun, doi:10.1038/s41467-019-12973-1
Zhang, Stacey, Agostino, Tugg, Marzok et al., Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection, Nat Rev Immunol, doi:10.1038/s41577-022-00813-1
Zhou, Dcosta, Landau, Tada, Resistance of SARS-coV-2 omicron BA.1 and BA.2 variants to vaccine-elicited sera and therapeutic monoclonal antibodies, Viruses, doi:10.3390/v14061334
Zhou, Duyvesteyn, Chen, Huang, Chen et al., Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient, Nat Struct Mol Biol, doi:10.1038/s41594-020-0480-y
Zhou, Georgiev, Wu, Yang, Dai et al., Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01, Science, doi:10.1126/science.1192819
Zhou, Song, Liu, Yuan, He et al., Broadly neutralizing anti-S2 antibodies protect against all three human betacoronaviruses that cause deadly disease, Immunity, doi:10.1016/j.immuni.2023.02.005
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
Zhou, Yuan, Song, Beutler, Shaabani et al., A human antibody reveals a conserved site on beta-coronavirus spike proteins and confers protection against SARS-CoV-2 infection, Sci Transl Med, doi:10.1126/scitranslmed.abi9215
Zhu, Liu, Bess, Jr, Chertova et al., Distribution and three-dimensional structure of AIDS virus envelope spikes, Nature, doi:10.1038/nature04817
DOI record:
{
"DOI": "10.3389/fimmu.2025.1733684",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2025.1733684",
"abstract": "<jats:p>Enveloped viruses such as Human Immunodeficiency Virus (HIV-1) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) have caused some of the deadliest pandemics in human history. These viruses utilize Class 1 viral fusion glycoproteins to bind their host receptor and subsequently fuse the virus and host cell membranes to mediate entry. Viral fusion glycoproteins are prominent antigens on the surface of virions and are essential for the virus life cycle. Therefore, they are a primary target for the humoral immune system and the basis for the design of vaccines. Antibodies which target viral fusion glycoproteins can neutralize viral infectivity and activate the immune system in several distinct ways. In this review, we compare mechanisms of how class 1 viral fusion glycoproteins mediate viral entry and cover diverse ways in which antibodies targeting these glycoproteins can neutralize viruses and activate the immune system to clear virus-infected cells.</jats:p>",
"alternative-id": [
"10.3389/fimmu.2025.1733684"
],
"article-number": "1733684",
"author": [
{
"affiliation": [],
"family": "Grunst",
"given": "Michael W.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Li",
"given": "Wenwei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mothes",
"given": "Walther",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T06:36:53Z",
"timestamp": 1767940613000
},
"deposited": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T06:36:55Z",
"timestamp": 1767940615000
},
"indexed": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T08:14:28Z",
"timestamp": 1767946468031,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
1,
9
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T00:00:00Z",
"timestamp": 1767916800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2025.1733684/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2026,
1,
9
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
9
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1056/NEJMc2206576",
"article-title": "Neutralization escape by SARS-coV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5",
"author": "Hachmann",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B1",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1101/2024.10.23.619754",
"article-title": "Enhanced immune evasion of SARS-CoV-2 variants KP.3.1.1 and XEC through N-terminal domain mutations",
"author": "Liu",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "B2",
"year": "2024"
},
{
"DOI": "10.15585/mmwr.mm7145a2",
"article-title": "Interim recommendations from the advisory committee on immunization practices for the use of bivalent booster doses of COVID-19 vaccines - United States, october 2022",
"author": "Rosenblum",
"doi-asserted-by": "publisher",
"journal-title": "MMWR Morb Mort Wkly Rep",
"key": "B3",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1186/s40794-020-00129-9",
"article-title": "COVID-19 in comparison with other emerging viral diseases: risk of geographic spread via travel",
"author": "Wilder-Smith",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Trop Dis Trav Med Vaccines",
"key": "B4",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1080/10409230802058320",
"article-title": "Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme",
"author": "White",
"doi-asserted-by": "publisher",
"first-page": "189",
"journal-title": "Crit Rev Biochem Mol Biol",
"key": "B5",
"volume": "43",
"year": "2008"
},
{
"DOI": "10.1038/nsmb.1456",
"article-title": "Viral membrane fusion",
"author": "Harrison",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B6",
"volume": "15",
"year": "2008"
},
{
"DOI": "10.1080/09687860400017784",
"article-title": "Class I and class II viral fusion protein structures reveal similar principles in membrane fusion",
"author": "Schibli",
"doi-asserted-by": "publisher",
"journal-title": "Mol Membr Biol",
"key": "B7",
"volume": "21",
"year": "2004"
},
{
"DOI": "10.1186/1742-4690-5-111",
"article-title": "Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm",
"author": "Melikyan",
"doi-asserted-by": "publisher",
"first-page": "111",
"journal-title": "Retrovirology",
"key": "B8",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.1016/j.tibs.2024.06.012",
"article-title": "Visualizing intermediate stages of viral membrane fusion by cryo-electron tomography",
"author": "Kephart",
"doi-asserted-by": "publisher",
"journal-title": "Trends Biochem Sci",
"key": "B9",
"volume": "49",
"year": "2024"
},
{
"DOI": "10.1038/312763a0",
"article-title": "The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus",
"author": "Dalgleish",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B10",
"volume": "312",
"year": "1984"
},
{
"DOI": "10.1038/nature02145",
"article-title": "Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B11",
"volume": "426",
"year": "2003"
},
{
"DOI": "10.1016/0092-8674(93)90260-W",
"article-title": "A spring-loaded mechanism for the conformational change of influenza hemagglutinin",
"author": "Carr",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "B12",
"volume": "73",
"year": "1993"
},
{
"DOI": "10.1083/jcb.200607083",
"article-title": "Membranes of the world unite",
"author": "Chernomordik",
"doi-asserted-by": "publisher",
"journal-title": "J Cell Biol",
"key": "B13",
"volume": "175",
"year": "2006"
},
{
"DOI": "10.1146/annurev.biochem.72.121801.161504",
"article-title": "Protein-lipid interplay in fusion and fission of biological membranes",
"author": "Chernomordik",
"doi-asserted-by": "publisher",
"first-page": "175",
"journal-title": "Annu Rev Biochem",
"key": "B14",
"volume": "72",
"year": "2003"
},
{
"DOI": "10.1038/nature13808",
"article-title": "Structure and immune recognition of trimeric pre-fusion HIV-1 Env",
"author": "Pancera",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B15",
"volume": "514",
"year": "2014"
},
{
"DOI": "10.1038/371037a0",
"article-title": "Structure of influenza haemagglutinin at the pH of membrane fusion",
"author": "Bullough",
"doi-asserted-by": "publisher",
"first-page": "37",
"journal-title": "Nature",
"key": "B16",
"volume": "371",
"year": "1994"
},
{
"DOI": "10.1038/s41586-023-06273-4",
"article-title": "Cryo-EM structure of SARS-CoV-2 postfusion spike in membrane",
"author": "Shi",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B17",
"volume": "619",
"year": "2023"
},
{
"DOI": "10.1126/science.adn5658",
"article-title": "Structure and inhibition of SARS-CoV-2 spike refolding in membranes",
"author": "Grunst",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B18",
"volume": "385",
"year": "2024"
},
{
"DOI": "10.1038/nmicrobiol.2016.50",
"article-title": "The hemifusion structure induced by influenza virus haemagglutinin is determined by physical properties of the target membranes",
"author": "Chlanda",
"doi-asserted-by": "publisher",
"first-page": "16050",
"journal-title": "Nat Microbiol",
"key": "B19",
"volume": "1",
"year": "2016"
},
{
"DOI": "10.1016/j.chemphyslip.2014.07.010",
"article-title": "Mechanics of membrane fusion/pore formation",
"author": "Fuhrmans",
"doi-asserted-by": "publisher",
"journal-title": "Chem Phys Lipids",
"key": "B20",
"volume": "185",
"year": "2015"
},
{
"DOI": "10.1073/pnas.121191898",
"article-title": "A quantitative model for membrane fusion based on low-energy intermediates",
"author": "Kuzmin",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B21",
"volume": "98",
"year": "2001"
},
{
"DOI": "10.3390/ijms21113875",
"article-title": "Continuum models of membrane fusion: evolution of the theory",
"author": "Akimov",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "B22",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1038/nsmb.1455",
"article-title": "Mechanics of membrane fusion",
"author": "Chernomordik",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B23",
"volume": "15",
"year": "2008"
},
{
"DOI": "10.1091/mbc.11.7.2359",
"article-title": "Reversible merger of membranes at the early stage of influenza hemagglutinin-mediated fusion",
"author": "Leikina",
"doi-asserted-by": "publisher",
"journal-title": "Mol Biol Cell",
"key": "B24",
"volume": "11",
"year": "2000"
},
{
"DOI": "10.1126/sciadv.abo3153",
"article-title": "Intermediates in SARS-CoV-2 spike-mediated cell entry",
"author": "Marcink",
"doi-asserted-by": "publisher",
"journal-title": "Sci Adv",
"key": "B25",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.7554/eLife.70362",
"article-title": "Sterically confined rearrangements of SARS-CoV-2 Spike protein control cell invasion",
"author": "Dodero-Rojas",
"doi-asserted-by": "publisher",
"journal-title": "Elife",
"key": "B26",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.7554/eLife.58411",
"article-title": "Electron tomography visualization of HIV-1 fusion with target cells using fusion inhibitors to trap the pre-hairpin intermediate",
"author": "Ladinsky",
"doi-asserted-by": "publisher",
"journal-title": "Elife",
"key": "B27",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1128/jvi.01432-24",
"article-title": "Electron tomography visualization of HIV-1 virions trapped by fusion inhibitors to host cells in infected tissues",
"author": "Ladinsky",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B28",
"volume": "98",
"year": "2024"
},
{
"DOI": "10.1038/nsmb.3271",
"article-title": "Cryomicroscopy provides structural snapshots of influenza virus membrane fusion",
"author": "Calder",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B29",
"volume": "23",
"year": "2016"
},
{
"DOI": "10.1038/nm0403-393",
"article-title": "HIV-1: nature’s master of disguise",
"author": "Mascola",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B30",
"volume": "9",
"year": "2003"
},
{
"DOI": "10.1038/s41579-022-00841-7",
"article-title": "SARS-CoV-2 variant biology: immune escape, transmission and fitness",
"author": "Carabelli",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Microbiol",
"key": "B31",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1038/nsmb.3291",
"article-title": "Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site",
"author": "Gristick",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B32",
"volume": "23",
"year": "2016"
},
{
"DOI": "10.1016/j.cell.2016.04.010",
"article-title": "Trimeric HIV-1-env structures define glycan shields from clades A, B and G",
"author": "Stewart-Jones",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "B33",
"volume": "165",
"year": "2016"
},
{
"DOI": "10.1038/s44298-024-00085-7",
"article-title": "Conserved role of spike S2 domain N-glycosylation across betacoronaviruses",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "4",
"journal-title": "NPJ Viruses",
"key": "B34",
"volume": "3",
"year": "2025"
},
{
"DOI": "10.3389/fimmu.2021.637651",
"article-title": "The case for S2: the potential benefits of the S2 subunit of the SARS-coV-2 spike protein as an immunogen in fighting the COVID-19 pandemic",
"author": "Shah",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B35",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fmicb.2022.855232",
"article-title": "HIV transmembrane glycoprotein conserved domains and genetic markers across HIV-1 and HIV-2 variants",
"author": "Valades-Alcaraz",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "B36",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2020.107583",
"article-title": "HIV-1 envelope and MPER antibody structures in lipid assemblies",
"author": "Rantalainen",
"doi-asserted-by": "publisher",
"first-page": "107583",
"journal-title": "Cell Rep",
"key": "B37",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1038/s41467-022-34008-y",
"article-title": "Dynamic HIV-1 spike motion creates vulnerability for its membrane-bound tripod to antibody attack",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "6393",
"journal-title": "Nat Commun",
"key": "B38",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1126/sciimmunol.aal2200",
"article-title": "Potent and broad HIV-neutralizing antibodies in memory B cells and plasma",
"author": "Williams",
"doi-asserted-by": "publisher",
"journal-title": "Sci Immunol",
"key": "B39",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1038/s41467-019-12973-1",
"article-title": "An MPER antibody neutralizes HIV-1 using germline features shared among donors",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "5389",
"journal-title": "Nat Commun",
"key": "B40",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1038/nature11544",
"article-title": "Broad and potent neutralization of HIV-1 by a gp41-specific human antibody",
"author": "Huang",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B41",
"volume": "491",
"year": "2012"
},
{
"DOI": "10.1126/science.280.5371.1884",
"article-title": "The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens",
"author": "Wyatt",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B42",
"volume": "280",
"year": "1998"
},
{
"DOI": "10.1038/s41586-019-1101-y",
"article-title": "Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET",
"author": "Lu",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B43",
"volume": "568",
"year": "2019"
},
{
"DOI": "10.1126/science.1254426",
"article-title": "Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions",
"author": "Munro",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B44",
"volume": "346",
"year": "2014"
},
{
"DOI": "10.1016/j.chom.2019.03.002",
"article-title": "An asymmetric opening of HIV-1 envelope mediates antibody-dependent cellular cytotoxicity",
"author": "Alsahafi",
"doi-asserted-by": "publisher",
"journal-title": "Cell Host Microbe",
"key": "B45",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1038/s41467-023-39262-2",
"article-title": "Antiviral HIV-1 SERINC restriction factors disrupt virus membrane asymmetry",
"author": "Leonhardt",
"doi-asserted-by": "publisher",
"first-page": "4368",
"journal-title": "Nat Commun",
"key": "B46",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41586-023-06762-6",
"article-title": "HIV-1 Env trimers asymmetrically engage CD4 receptors in membranes",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B47",
"volume": "623",
"year": "2023"
},
{
"DOI": "10.1038/s41586-023-06639-8",
"article-title": "Intermediate conformations of CD4-bound HIV-1 Env heterotrimers",
"author": "Dam",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B48",
"volume": "623",
"year": "2023"
},
{
"DOI": "10.1038/312767a0",
"article-title": "T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV",
"author": "Klatzmann",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B49",
"volume": "312",
"year": "1984"
},
{
"DOI": "10.1126/science.3001934",
"article-title": "Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule",
"author": "McDougal",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B50",
"volume": "231",
"year": "1986"
},
{
"DOI": "10.1016/0092-8674(86)90590-8",
"article-title": "The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain",
"author": "Maddon",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "B51",
"volume": "47",
"year": "1986"
},
{
"DOI": "10.1073/pnas.1615939113",
"article-title": "Cryo-EM structure of a CD4-bound open HIV-1 envelope trimer reveals structural rearrangements of the gp120 V1V2 loop",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B52",
"volume": "113",
"year": "2016"
},
{
"DOI": "10.1038/nature23010",
"article-title": "Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike",
"author": "Ozorowski",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B53",
"volume": "547",
"year": "2017"
},
{
"DOI": "10.1126/science.1261064",
"article-title": "Structural biology. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine",
"author": "Qin",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B54",
"volume": "347",
"year": "2015"
},
{
"DOI": "10.1038/s41586-018-0804-9",
"article-title": "Structural basis of coreceptor recognition by HIV-1 envelope spike",
"author": "Shaik",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B55",
"volume": "565",
"year": "2019"
},
{
"DOI": "10.1038/s41594-018-0113-x",
"article-title": "A dynamic three-step mechanism drives the HIV-1 pre-fusion reaction",
"author": "Iliopoulou",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B56",
"volume": "25",
"year": "2018"
},
{
"DOI": "10.1126/science.272.5263.872",
"article-title": "HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor",
"author": "Feng",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B57",
"volume": "272",
"year": "1996"
},
{
"DOI": "10.1038/381667a0",
"article-title": "HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5",
"author": "Dragic",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B58",
"volume": "381",
"year": "1996"
},
{
"DOI": "10.1038/381661a0",
"article-title": "Identification of a major co-receptor for primary isolates of HIV-1",
"author": "Deng",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B59",
"volume": "381",
"year": "1996"
},
{
"DOI": "10.1016/S0092-8674(00)81313-6",
"article-title": "The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates",
"author": "Choe",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "B60",
"volume": "85",
"year": "1996"
},
{
"DOI": "10.1084/jem.185.4.621",
"article-title": "Change in coreceptor use correlates with disease progression in HIV-1–infected individuals",
"author": "Connor",
"doi-asserted-by": "publisher",
"journal-title": "J Exp Med",
"key": "B61",
"volume": "185",
"year": "1997"
},
{
"DOI": "10.1146/annurev.immunol.17.1.657",
"article-title": "Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease",
"author": "Berger",
"doi-asserted-by": "publisher",
"first-page": "657",
"journal-title": "Annu Rev Immunol",
"key": "B62",
"volume": "17",
"year": "1999"
},
{
"DOI": "10.1038/s41467-019-08825-7",
"article-title": "A sequestered fusion peptide in the structure of an HIV-1 transmitted founder envelope trimer",
"author": "Ananthaswamy",
"doi-asserted-by": "publisher",
"first-page": "873",
"journal-title": "Nat Commun",
"key": "B63",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1038/s41467-019-08738-5",
"article-title": "Capturing the inherent structural dynamics of the HIV-1 envelope glycoprotein fusion peptide",
"author": "Kumar",
"doi-asserted-by": "publisher",
"first-page": "763",
"journal-title": "Nat Commun",
"key": "B64",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1038/s41594-019-0344-5",
"article-title": "Asymmetric opening of HIV-1 Env bound to CD4 and a coreceptor-mimicking antibody",
"author": "Yang",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B65",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1038/s41467-025-59721-2",
"article-title": "Conformational trajectory of the HIV-1 fusion peptide during CD4-induced envelope opening",
"author": "Thakur",
"doi-asserted-by": "publisher",
"first-page": "4595",
"journal-title": "Nat Commun",
"key": "B66",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.1371/journal.ppat.1000880",
"article-title": "Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions",
"author": "Buzon",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B67",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.1038/s41467-017-00515-6",
"article-title": "Envelope glycoprotein mobility on HIV-1 particles depends on the virus maturation state",
"author": "Chojnacki",
"doi-asserted-by": "publisher",
"first-page": "545",
"journal-title": "Nat Commun",
"key": "B68",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1126/science.1226359",
"article-title": "Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy",
"author": "Chojnacki",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B69",
"volume": "338",
"year": "2012"
},
{
"DOI": "10.1016/j.cell.2022.01.013",
"article-title": "Cryo-ET of Env on intact HIV virions reveals structural variation and positioning on the Gag lattice",
"author": "Prasad",
"doi-asserted-by": "publisher",
"first-page": "641",
"journal-title": "Cell",
"key": "B70",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1038/s41586-025-08624-9",
"article-title": "The conserved HIV-1 spacer peptide 2 triggers matrix lattice maturation",
"author": "Stacey",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B71",
"volume": "640",
"year": "2025"
},
{
"DOI": "10.1371/journal.ppat.1004595",
"article-title": "Different infectivity of HIV-1 strains is linked to number of envelope trimers required for entry",
"author": "Brandenberg",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B72",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1128/mbio.02754-24",
"article-title": "Inter-protomer opening cooperativity of envelope trimers positively correlates with HIV-1 entry stoichiometry",
"author": "Katte",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "B73",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"article-title": "Structure, function, and antigenicity of the SARS-coV-2 spike glycoprotein",
"author": "Walls",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Cell",
"key": "B74",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A pneumonia outbreak associated with a new coronavirus of probable bat origin",
"author": "Zhou",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B75",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1073/pnas.0409465102",
"article-title": "Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry",
"author": "Hofmann",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B76",
"volume": "102",
"year": "2005"
},
{
"DOI": "10.1038/357420a0",
"article-title": "Human aminopeptidase N is a receptor for human coronavirus 229E",
"author": "Yeager",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B77",
"volume": "357",
"year": "1992"
},
{
"DOI": "10.7554/eLife.51230",
"article-title": "The human coronavirus HCoV-229E S-protein structure and receptor binding",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Elife",
"key": "B78",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1038/s41467-017-01706-x",
"article-title": "Receptor-binding loops in alphacoronavirus adaptation and evolution",
"author": "Wong",
"doi-asserted-by": "publisher",
"first-page": "1735",
"journal-title": "Nat Commun",
"key": "B79",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1038/nature12328",
"article-title": "Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26",
"author": "Lu",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B80",
"volume": "500",
"year": "2013"
},
{
"DOI": "10.1038/s41586-023-06761-7",
"article-title": "TMPRSS2 is a functional receptor for human coronavirus HKU1",
"author": "Saunders",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B81",
"volume": "624",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2024.06.006",
"article-title": "Human coronavirus HKU1 recognition of the TMPRSS2 host receptor",
"author": "McCallum",
"doi-asserted-by": "publisher",
"first-page": "4231",
"journal-title": "Cell",
"key": "B82",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1016/j.cell.2024.06.007",
"article-title": "Structural basis of TMPRSS2 zymogen activation and recognition by the HKU1 seasonal coronavirus",
"author": "Fernandez",
"doi-asserted-by": "publisher",
"first-page": "4246",
"journal-title": "Cell",
"key": "B83",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1016/j.cell.2024.06.016",
"article-title": "TMPRSS2 and glycan receptors synergistically facilitate coronavirus entry",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "4261",
"journal-title": "Cell",
"key": "B84",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1073/pnas.1712592114",
"article-title": "Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B85",
"volume": "114",
"year": "2017"
},
{
"DOI": "10.1016/j.cell.2022.05.019",
"article-title": "Structure, receptor recognition, and antigenicity of the human coronavirus CCoV-HuPn-2018 spike glycoprotein",
"author": "Tortorici",
"doi-asserted-by": "publisher",
"first-page": "2279",
"journal-title": "Cell",
"key": "B86",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1038/s41594-019-0334-7",
"article-title": "Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors",
"author": "Park",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B87",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1038/s41586-023-06599-z",
"article-title": "Sialoglycan binding triggers spike opening in a human coronavirus",
"author": "Pronker",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B88",
"volume": "624",
"year": "2023"
},
{
"DOI": "10.1038/s41589-021-00924-1",
"article-title": "Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2",
"author": "Nguyen",
"doi-asserted-by": "publisher",
"first-page": "81",
"journal-title": "Nat Chem Biol",
"key": "B89",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1038/s41594-019-0233-y",
"article-title": "Structural basis for human coronavirus attachment to sialic acid receptors",
"author": "Tortorici",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B90",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1038/ncomms15092",
"article-title": "Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains",
"author": "Yuan",
"doi-asserted-by": "publisher",
"first-page": "15092",
"journal-title": "Nat Commun",
"key": "B91",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1126/science.abb2507",
"article-title": "Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation",
"author": "Wrapp",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B92",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1101/2020.06.27.174979",
"article-title": "Structures and distributions of SARS-CoV-2 spike proteins on intact virions",
"author": "Ke",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B93",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.11.001",
"article-title": "Real-time conformational dynamics of SARS-coV-2 spikes on virus particles",
"author": "Lu",
"doi-asserted-by": "publisher",
"first-page": "880",
"journal-title": "Cell Host Microbe",
"key": "B94",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1016/j.str.2024.09.008",
"article-title": "Conformational dynamics of SARS-CoV-2 Omicron spike trimers during fusion activation at single molecule resolution",
"author": "Dey",
"doi-asserted-by": "publisher",
"first-page": "1910",
"journal-title": "Structure",
"key": "B95",
"volume": "32",
"year": "2024"
},
{
"DOI": "10.7554/eLife.75433",
"article-title": "Conformational dynamics and allosteric modulation of the SARS-CoV-2 spike",
"author": "Diaz-Salinas",
"doi-asserted-by": "publisher",
"journal-title": "Elife",
"key": "B96",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1126/sciadv.adk4920",
"article-title": "Single-molecule imaging reveals allosteric stimulation of SARS-CoV-2 spike receptor binding domain by host sialic acid",
"author": "Diaz-Salinas",
"doi-asserted-by": "publisher",
"journal-title": "Sci Adv",
"key": "B97",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1038/s41467-023-38251-9",
"article-title": "Detergent modulates the conformational equilibrium of SARS-CoV-2 Spike during cryo-EM structural determination",
"author": "Egri",
"doi-asserted-by": "publisher",
"first-page": "2527",
"journal-title": "Nat Commun",
"key": "B98",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.celrep.2022.110694",
"article-title": "Dynamic Ca(2+) sensitivity stimulates the evolved SARS-CoV-2 spike strain-mediated membrane fusion for enhanced entry",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "110694",
"journal-title": "Cell Rep",
"key": "B99",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1128/mbio.03227-21",
"article-title": "SARS-coV-2 variants increase kinetic stability of open spike conformations as an evolutionary strategy",
"author": "Yang",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "B100",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-28882-9",
"article-title": "Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain",
"author": "Ye",
"doi-asserted-by": "publisher",
"first-page": "1214",
"journal-title": "Nat Commun",
"key": "B101",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2852-1",
"article-title": "SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies",
"author": "Barnes",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B102",
"volume": "588",
"year": "2020"
},
{
"DOI": "10.1126/science.abd5223",
"article-title": "In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges",
"author": "Turonova",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B103",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.09.018",
"article-title": "Molecular architecture of the SARS-coV-2 virus",
"author": "Yao",
"doi-asserted-by": "publisher",
"first-page": "730",
"journal-title": "Cell",
"key": "B104",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1038/s41467-023-42836-9",
"article-title": "Structural insights into the modulation of coronavirus spike tilting and infectivity by hinge glycans",
"author": "Chmielewski",
"doi-asserted-by": "publisher",
"first-page": "7175",
"journal-title": "Nat Commun",
"key": "B105",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.bpj.2020.10.036",
"article-title": "The flexibility of ACE2 in the context of SARS-CoV-2 infection",
"author": "Barros",
"doi-asserted-by": "publisher",
"journal-title": "Biophys J",
"key": "B106",
"volume": "120",
"year": "2021"
},
{
"DOI": "10.1074/jbc.M112.398842",
"article-title": "The X-ray crystal structure of human aminopeptidase N reveals a novel dimer and the basis for peptide processing",
"author": "Wong",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "B107",
"volume": "287",
"year": "2012"
},
{
"DOI": "10.1074/jbc.M405001200",
"article-title": "Crystal structure of CD26/dipeptidyl-peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface",
"author": "Weihofen",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "B108",
"volume": "279",
"year": "2004"
},
{
"DOI": "10.1038/s41467-020-15562-9",
"article-title": "Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV",
"author": "Ou",
"doi-asserted-by": "publisher",
"first-page": "1620",
"journal-title": "Nat Commun",
"key": "B109",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"article-title": "A multibasic cleavage site in the spike protein of SARS-coV-2 is essential for infection of human lung cells",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "779",
"journal-title": "Mol Cell",
"key": "B110",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2003138117",
"article-title": "Cell entry mechanisms of SARS-CoV-2",
"author": "Shang",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B111",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1407087111",
"article-title": "Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein",
"author": "Millet",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B112",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1038/s41564-021-00908-w",
"article-title": "The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets",
"author": "Peacock",
"doi-asserted-by": "publisher",
"first-page": "899",
"journal-title": "Nat Microbiol",
"key": "B113",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1073/pnas.1608147113",
"article-title": "Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism",
"author": "Park",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B114",
"volume": "113",
"year": "2016"
},
{
"DOI": "10.1016/j.virol.2017.12.015",
"article-title": "Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells",
"author": "Millet",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Virology",
"key": "B115",
"volume": "517",
"year": "2018"
},
{
"DOI": "10.1016/j.cell.2025.01.012",
"article-title": "Early fusion intermediate of ACE2-using coronavirus spike acting as an antiviral target",
"author": "Xing",
"doi-asserted-by": "publisher",
"first-page": "1297",
"journal-title": "Cell",
"key": "B116",
"volume": "188",
"year": "2025"
},
{
"DOI": "10.1038/s41586-020-2772-0",
"article-title": "Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion",
"author": "Benton",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B117",
"volume": "588",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "B118",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41467-025-60406-z",
"article-title": "Unveiling the structural spectrum of SARS-CoV-2 fusion by in situ cryo-ET",
"author": "Akil",
"doi-asserted-by": "publisher",
"first-page": "5150",
"journal-title": "Nat Commun",
"key": "B119",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.1038/s41467-020-17371-6",
"article-title": "Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein",
"author": "Fan",
"doi-asserted-by": "publisher",
"first-page": "3618",
"journal-title": "Nat Commun",
"key": "B120",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.15252/embj.2021107821",
"article-title": "TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells",
"author": "Koch",
"doi-asserted-by": "publisher",
"journal-title": "EMBO J",
"key": "B121",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1016/j.celrep.2025.115916",
"article-title": "Single-molecule imaging prefusion intermediate conformations of MERS-CoV spike trimers in membrane during entry",
"author": "Dey",
"doi-asserted-by": "publisher",
"first-page": "115916",
"journal-title": "Cell Rep",
"key": "B122",
"volume": "44",
"year": "2025"
},
{
"DOI": "10.1111/imr.13431",
"article-title": "Structural immunology of SARS-coV-2",
"author": "Yuan",
"doi-asserted-by": "publisher",
"journal-title": "Immunol Rev",
"key": "B123",
"year": "2024"
},
{
"DOI": "10.1038/s41590-018-0235-7",
"article-title": "Recent progress in broadly neutralizing antibodies to HIV",
"author": "Sok",
"doi-asserted-by": "publisher",
"journal-title": "Nat Immunol",
"key": "B124",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1038/s41594-020-0452-2",
"article-title": "Subnanometer structures of HIV-1 envelope trimers on aldrithiol-2-inactivated virus particles",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B125",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1002/pro.3943",
"article-title": "UCSF ChimeraX: Structure visualization for researchers, educators, and developers",
"author": "Pettersen",
"doi-asserted-by": "publisher",
"first-page": "70",
"journal-title": "Protein Sci",
"key": "B126",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1002/pro.3235",
"article-title": "UCSF ChimeraX: Meeting modern challenges in visualization and analysis",
"author": "Goddard",
"doi-asserted-by": "publisher",
"first-page": "14",
"journal-title": "Protein Sci",
"key": "B127",
"volume": "27",
"year": "2018"
},
{
"DOI": "10.1038/s41577-023-00858-w",
"article-title": "Antiviral neutralizing antibodies: from in vitro to in vivo activity",
"author": "Burton",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B128",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.celrep.2021.110210",
"article-title": "Structural basis and mode of action for two broadly neutralizing antibodies against SARS-CoV-2 emerging variants of concern",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "110210",
"journal-title": "Cell Rep",
"key": "B129",
"volume": "38",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.09.037",
"article-title": "Mapping neutralizing and immunodominant sites on the SARS-coV-2 spike receptor-binding domain by structure-guided high-resolution serology",
"author": "Piccoli",
"doi-asserted-by": "publisher",
"first-page": "1024",
"journal-title": "Cell",
"key": "B130",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-20501-9",
"article-title": "Antibody neutralization of SARS-CoV-2 through ACE2 receptor mimicry",
"author": "Ge",
"doi-asserted-by": "publisher",
"first-page": "250",
"journal-title": "Nat Commun",
"key": "B131",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41422-021-00487-9",
"article-title": "Structural basis for bivalent binding and inhibition of SARS-CoV-2 infection by human potent neutralizing antibodies",
"author": "Yan",
"doi-asserted-by": "publisher",
"journal-title": "Cell Res",
"key": "B132",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1016/j.immuni.2016.10.027",
"article-title": "Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth",
"author": "Huang",
"doi-asserted-by": "publisher",
"journal-title": "Immunity",
"key": "B133",
"volume": "45",
"year": "2016"
},
{
"DOI": "10.1126/science.1187659",
"article-title": "Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B134",
"volume": "329",
"year": "2010"
},
{
"DOI": "10.1126/science.1192819",
"article-title": "Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01",
"author": "Zhou",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B135",
"volume": "329",
"year": "2010"
},
{
"DOI": "10.1126/science.1207227",
"article-title": "Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding",
"author": "Scheid",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B136",
"volume": "333",
"year": "2011"
},
{
"DOI": "10.1038/nature14411",
"article-title": "Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117",
"author": "Caskey",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B137",
"volume": "522",
"year": "2015"
},
{
"DOI": "10.1038/nature18929",
"article-title": "HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption",
"author": "Scheid",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B138",
"volume": "535",
"year": "2016"
},
{
"DOI": "10.1056/NEJMoa2031738",
"article-title": "Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition",
"author": "Corey",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B139",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1128/JVI.00754-11",
"article-title": "Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B140",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.1126/science.aad2450",
"article-title": "Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer",
"author": "Lee",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B141",
"volume": "351",
"year": "2016"
},
{
"DOI": "10.1038/s41594-022-00852-1",
"article-title": "Cryo-EM structures of prefusion SIV envelope trimer",
"author": "Gorman",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B142",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1128/mBio.01255-19",
"article-title": "Differences in the binding affinity of an HIV-1 V2 apex-specific antibody for the SIV",
"author": "von Bredow",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "B143",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.immuni.2017.03.017",
"article-title": "A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic beta-Hairpin Structure",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "690",
"journal-title": "Immunity",
"key": "B144",
"volume": "46",
"year": "2017"
},
{
"DOI": "10.1016/j.celrep.2020.03.052",
"article-title": "Structure of super-potent antibody CAP256-VRC26.25 in complex with HIV-1 envelope reveals a combined mode of trimer-apex recognition",
"author": "Gorman",
"doi-asserted-by": "publisher",
"first-page": "107488",
"journal-title": "Cell Rep",
"key": "B145",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00410-17",
"article-title": "Plasticity and epitope exposure of the HIV-1 envelope trimer",
"author": "Powell",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B146",
"volume": "91",
"year": "2017"
},
{
"DOI": "10.1371/journal.ppat.1011819",
"article-title": "Potent antibody-dependent cellular cytotoxicity of a V2-specific antibody is not sufficient for protection of macaques against SIV challenge",
"author": "Grunst",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B147",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.1126/science.abh2315",
"article-title": "Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: A global consortium study",
"author": "Hastie",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B148",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1038/s41577-022-00784-3",
"article-title": "Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B149",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1038/s41586-020-2571-7",
"article-title": "Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike",
"author": "Liu",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B150",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1038/s41467-024-54746-5",
"article-title": "Exploring distinct modes of inter-spike cross-linking for enhanced neutralization by SARS-CoV-2 antibodies",
"author": "Nan",
"doi-asserted-by": "publisher",
"first-page": "10578",
"journal-title": "Nat Commun",
"key": "B151",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1038/s41467-023-35949-8",
"article-title": "Structural basis for a conserved neutralization epitope on the receptor-binding domain of SARS-CoV-2",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "311",
"journal-title": "Nat Commun",
"key": "B152",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41594-020-0480-y",
"article-title": "Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient",
"author": "Zhou",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B153",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1126/science.abb7269",
"article-title": "A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV",
"author": "Yuan",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B154",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1126/science.abq0839",
"article-title": "Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models",
"author": "Cohen",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B155",
"volume": "377",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2024.07.052",
"article-title": "Mosaic sarbecovirus nanoparticles elicit cross-reactive responses in pre-vaccinated animals",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "5554",
"journal-title": "Cell",
"key": "B156",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1038/s41467-022-28307-7",
"article-title": "Broadly neutralizing anti-HIV-1 antibodies tether viral particles at the surface of infected cells",
"author": "Dufloo",
"doi-asserted-by": "publisher",
"first-page": "630",
"journal-title": "Nat Commun",
"key": "B157",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.immuni.2023.09.003",
"article-title": "Antibodies targeting a quaternary site on SARS-CoV-2 spike glycoprotein prevent viral receptor engagement by conformational locking",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "2442",
"journal-title": "Immunity",
"key": "B158",
"volume": "56",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2021.03.028",
"article-title": "N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2",
"author": "McCallum",
"doi-asserted-by": "publisher",
"first-page": "2332",
"journal-title": "Cell",
"key": "B159",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.03.029",
"article-title": "Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein",
"author": "Suryadevara",
"doi-asserted-by": "publisher",
"first-page": "2316",
"journal-title": "Cell",
"key": "B160",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.3390/vaccines11030545",
"article-title": "Significance of conserved regions in coronavirus spike protein for developing a novel vaccine against SARS-coV-2 infection",
"author": "Olukitibi",
"doi-asserted-by": "publisher",
"journal-title": "Vaccines (Basel)",
"key": "B161",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1371/journal.pbio.3000114",
"article-title": "CD4 occupancy triggers sequential pre-fusion conformational states of the HIV-1 envelope trimer with relevance for broadly neutralizing antibody activity",
"author": "Ivan",
"doi-asserted-by": "publisher",
"journal-title": "PloS Biol",
"key": "B162",
"volume": "17",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2017.01154",
"article-title": "Immunologic insights on the membrane proximal external region: A major human immunodeficiency virus type-1 vaccine target",
"author": "Molinos-Albert",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B163",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1038/nsmb0510-543",
"article-title": "HIV-1 autoreactive antibodies: are they good or bad for HIV-1 prevention",
"author": "Haynes",
"doi-asserted-by": "publisher",
"journal-title": "Nat Struct Mol Biol",
"key": "B164",
"volume": "17",
"year": "2010"
},
{
"DOI": "10.3389/fimmu.2021.708227",
"article-title": "To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1",
"author": "Griffith",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B165",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41467-025-65866-x",
"article-title": "The asymmetric opening of HIV-1 Env by a potent CD4 mimetic enables anti-coreceptor binding site antibodies to mediate ADCC",
"author": "Richard",
"doi-asserted-by": "publisher",
"first-page": "10419",
"journal-title": "Nat Commun",
"key": "B166",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.1073/pnas.0908713106",
"article-title": "Role of HIV membrane in neutralization by two broadly neutralizing antibodies",
"author": "Alam",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B167",
"volume": "106",
"year": "2009"
},
{
"DOI": "10.1126/science.1111781",
"article-title": "Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies",
"author": "Haynes",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B168",
"volume": "308",
"year": "2005"
},
{
"DOI": "10.1086/595013",
"article-title": "Anticardiolipin antibodies in HIV infection are independently associated with antibodies to the membrane proximal external region of gp41 and with cell-associated HIV DNA and immune activation",
"author": "Martinez",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "B169",
"volume": "48",
"year": "2009"
},
{
"DOI": "10.1073/pnas.0912914107",
"article-title": "Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance",
"author": "Verkoczy",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B170",
"volume": "107",
"year": "2010"
},
{
"DOI": "10.4049/jimmunol.1301285",
"article-title": "Immune tolerance negatively regulates B cells in knock-in mice expressing broadly neutralizing HIV antibody 4E10",
"author": "Doyle-Cooper",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B171",
"volume": "191",
"year": "2013"
},
{
"DOI": "10.4049/jimmunol.1300770",
"article-title": "Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B172",
"volume": "191",
"year": "2013"
},
{
"DOI": "10.1016/j.cell.2024.04.033",
"article-title": "Vaccine induction of heterologous HIV-1-neutralizing antibody B cell lineages in humans",
"author": "Williams",
"doi-asserted-by": "publisher",
"first-page": "2919",
"journal-title": "Cell",
"key": "B173",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1016/j.cell.2024.10.003",
"article-title": "Potent and broad HIV-1 neutralization in fusion peptide-primed SHIV-infected macaques",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "7214",
"journal-title": "Cell",
"key": "B174",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1126/science.aae0474",
"article-title": "Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody",
"author": "Kong",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B175",
"volume": "352",
"year": "2016"
},
{
"DOI": "10.1038/s41598-020-59711-y",
"article-title": "Preclinical development of a fusion peptide conjugate as an HIV vaccine immunogen",
"author": "Ou",
"doi-asserted-by": "publisher",
"first-page": "3032",
"journal-title": "Sci Rep",
"key": "B176",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41591-018-0042-6",
"article-title": "Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1",
"author": "Xu",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B177",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.1016/j.chom.2019.04.011",
"article-title": "Conformational plasticity in the HIV-1 fusion peptide facilitates recognition by broadly neutralizing antibodies",
"author": "Yuan",
"doi-asserted-by": "publisher",
"first-page": "873",
"journal-title": "Cell Host Microbe",
"key": "B178",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1126/scitranslmed.adh9039",
"article-title": "Antibodies targeting the fusion peptide on the HIV envelope provide protection to rhesus macaques against mucosal SHIV challenge",
"author": "Pegu",
"doi-asserted-by": "publisher",
"journal-title": "Sci Transl Med",
"key": "B179",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1111/imr.12507",
"article-title": "The HIV-1 envelope glycoprotein structure: nailing down a moving target",
"author": "Ward",
"doi-asserted-by": "publisher",
"first-page": "21",
"journal-title": "Immunol Rev",
"key": "B180",
"volume": "275",
"year": "2017"
},
{
"DOI": "10.1080/22221751.2023.2220582",
"article-title": "SARS-CoV-2 spike S2-specific neutralizing antibodies",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "2220582",
"journal-title": "Emerg Microbes Infect",
"key": "B181",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7104e3",
"article-title": "Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION network, 10 states, august 2021–january 2022",
"author": "Thompson",
"doi-asserted-by": "crossref",
"journal-title": "Morb Mortality Wkly Rep",
"key": "B182",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-1175516/v1",
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies",
"author": "VanBlargan",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B183",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-1168453/v1",
"article-title": "mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant",
"author": "Gruell",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B184",
"year": "2022"
},
{
"DOI": "10.3390/v14061334",
"article-title": "Resistance of SARS-coV-2 omicron BA.1 and BA.2 variants to vaccine-elicited sera and therapeutic monoclonal antibodies",
"author": "Zhou",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B185",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04980-y",
"article-title": "BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection",
"author": "Cao",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B186",
"year": "2022"
},
{
"DOI": "10.1126/science.abg4493",
"article-title": "The emerging plasticity of SARS-CoV-2",
"author": "McCormick",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B187",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1126/science.abd4250",
"article-title": "Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity",
"author": "Shrock",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B188",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2021.108915",
"article-title": "Linear epitope landscape of the SARS-CoV-2 Spike protein constructed from 1,051 COVID-19 patients",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "108915",
"journal-title": "Cell Rep",
"key": "B189",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1038/s41423-020-00523-5",
"article-title": "Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Cell Mol Immunol",
"key": "B190",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1126/science.abe1107",
"article-title": "Preexisting and de novo humoral immunity to SARS-CoV-2 in humans",
"author": "Ng",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B191",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-23074-3",
"article-title": "Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection",
"author": "Song",
"doi-asserted-by": "publisher",
"first-page": "2938",
"journal-title": "Nat Commun",
"key": "B192",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1371/journal.ppat.1012383",
"article-title": "The S2 subunit of spike encodes diverse targets for functional antibody responses to SARS-CoV-2",
"author": "Guenthoer",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B193",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.1016/j.celrep.2021.109604",
"article-title": "Convergent antibody responses to the SARS-CoV-2 spike protein in convalescent and vaccinated individuals",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "109604",
"journal-title": "Cell Rep",
"key": "B194",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1016/j.str.2024.08.022",
"article-title": "Discovery and characterization of a pan-betacoronavirus S2-binding antibody",
"author": "Johnson",
"doi-asserted-by": "publisher",
"first-page": "1893",
"journal-title": "Structure",
"key": "B195",
"volume": "32",
"year": "2024"
},
{
"DOI": "10.1016/j.immuni.2023.02.005",
"article-title": "Broadly neutralizing anti-S2 antibodies protect against all three human betacoronaviruses that cause deadly disease",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "669",
"journal-title": "Immunity",
"key": "B196",
"volume": "56",
"year": "2023"
},
{
"DOI": "10.1126/scitranslmed.abi9215",
"article-title": "A human antibody reveals a conserved site on beta-coronavirus spike proteins and confers protection against SARS-CoV-2 infection",
"author": "Zhou",
"doi-asserted-by": "publisher",
"journal-title": "Sci Transl Med",
"key": "B197",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2022.10.010",
"article-title": "Rare, convergent antibodies targeting the stem helix broadly neutralize diverse betacoronaviruses",
"author": "Dacon",
"doi-asserted-by": "publisher",
"journal-title": "Cell Host Microbe",
"key": "B198",
"year": "2022"
},
{
"DOI": "10.1126/science.abj3321",
"article-title": "Broad betacoronavirus neutralization by a stem helix-specific human antibody",
"author": "Pinto",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B199",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1016/j.str.2022.06.004",
"article-title": "Vaccine-elicited murine antibody WS6 neutralizes diverse beta-coronaviruses by recognizing a helical stem supersite of vulnerability",
"author": "Shi",
"doi-asserted-by": "publisher",
"first-page": "1233",
"journal-title": "Structure",
"key": "B200",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2021.109929",
"article-title": "Stabilized coronavirus spike stem elicits a broadly protective antibody",
"author": "Hsieh",
"doi-asserted-by": "publisher",
"first-page": "109929",
"journal-title": "Cell Rep",
"key": "B201",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1038/s42003-022-03262-7",
"article-title": "Structural definition of a pan-sarbecovirus neutralizing epitope on the spike S2 subunit",
"author": "Hurlburt",
"doi-asserted-by": "publisher",
"first-page": "342",
"journal-title": "Commun Biol",
"key": "B202",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2021.109353",
"article-title": "Isolation and characterization of cross-neutralizing coronavirus antibodies from COVID-19+ subjects",
"author": "Jennewein",
"doi-asserted-by": "publisher",
"first-page": "109353",
"journal-title": "Cell Rep",
"key": "B203",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1038/s41467-023-43638-9",
"article-title": "Engineered immunogens to elicit antibodies against conserved coronavirus epitopes",
"author": "Kapingidza",
"doi-asserted-by": "publisher",
"first-page": "7897",
"journal-title": "Nat Commun",
"key": "B204",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1126/science.abq3773",
"article-title": "Broadly neutralizing antibodies target the coronavirus fusion peptide",
"author": "Dacon",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B205",
"volume": "377",
"year": "2022"
},
{
"DOI": "10.1038/s41564-022-01155-3",
"article-title": "Neutralization mechanism of a human antibody with pan-coronavirus reactivity including SARS-CoV-2",
"author": "Sun",
"doi-asserted-by": "publisher",
"journal-title": "Nat Microbiol",
"key": "B206",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1126/science.abq2679",
"article-title": "ACE2-binding exposes the SARS-CoV-2 fusion peptide to broadly neutralizing coronavirus antibodies",
"author": "Low",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B207",
"volume": "377",
"year": "2022"
},
{
"DOI": "10.7554/eLife.73490.sa2",
"article-title": "Comprehensive characterization of the antibody responses to SARS-CoV-2 Spike protein finds additional vaccine-induced epitopes beyond those for mild infection",
"author": "Garrett",
"doi-asserted-by": "publisher",
"journal-title": "Elife",
"key": "B208",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2015.01.016",
"article-title": "Intra-spike crosslinking overcomes antibody evasion by HIV-1",
"author": "Galimidi",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "B209",
"volume": "160",
"year": "2015"
},
{
"DOI": "10.1038/nature04817",
"article-title": "Distribution and three-dimensional structure of AIDS virus envelope spikes",
"author": "Zhu",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B210",
"volume": "441",
"year": "2006"
},
{
"DOI": "10.1371/journal.ppat.1000908",
"article-title": "Few and far between: how HIV may be evading antibody avidity",
"author": "Klein",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B211",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.1126/science.1213256",
"article-title": "A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield",
"author": "Pejchal",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B212",
"volume": "334",
"year": "2011"
},
{
"DOI": "10.1073/pnas.0811427106",
"article-title": "Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10",
"author": "Klein",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B213",
"volume": "106",
"year": "2009"
},
{
"DOI": "10.1016/j.chom.2023.06.006",
"article-title": "Anti-V1/V3-glycan broadly HIV-1 neutralizing antibodies in a post-treatment controller",
"author": "Molinos-Albert",
"doi-asserted-by": "publisher",
"first-page": "1275",
"journal-title": "Cell Host Microbe",
"key": "B214",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1128/JVI.78.19.10724-10737.2004",
"article-title": "Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope",
"author": "Ofek",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B215",
"volume": "78",
"year": "2004"
},
{
"DOI": "10.1038/s41422-022-00684-0",
"article-title": "A broadly neutralizing antibody against SARS-CoV-2 Omicron variant infection exhibiting a novel trimer dimer conformation in spike protein binding",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Cell Res",
"key": "B216",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2024.09.043",
"article-title": "Adaptive multi-epitope targeting and avidity-enhanced nanobody platform for ultrapotent, durable antiviral therapy",
"author": "Xiang",
"doi-asserted-by": "publisher",
"first-page": "6966",
"journal-title": "Cell",
"key": "B217",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1111/imcb.12324",
"article-title": "Antibody-mediated complement activation in pathology and protection",
"author": "Goldberg",
"doi-asserted-by": "publisher",
"journal-title": "Immunol Cell Biol",
"key": "B218",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-00410-0",
"article-title": "The role of IgG Fc receptors in antibody-dependent enhancement",
"author": "Bournazos",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B219",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1111/imr.12350",
"article-title": "Mouse and human FcR effector functions",
"author": "Bruhns",
"doi-asserted-by": "publisher",
"first-page": "25",
"journal-title": "Immunol Rev",
"key": "B220",
"volume": "268",
"year": "2015"
},
{
"DOI": "10.4049/jimmunol.2000501",
"article-title": "Functional interactions of common allotypes of rhesus macaque fcγR2A and fcγR3A with human and macaque igG subclasses",
"author": "Grunst",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B221",
"volume": "205",
"year": "2020"
},
{
"DOI": "10.1182/blood-2008-09-179754",
"article-title": "Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses",
"author": "Bruhns",
"doi-asserted-by": "publisher",
"journal-title": "Blood",
"key": "B222",
"volume": "113",
"year": "2009"
},
{
"DOI": "10.1038/nri2206",
"article-title": "Fcgamma receptors as regulators of immune responses",
"author": "Nimmerjahn",
"doi-asserted-by": "publisher",
"first-page": "34",
"journal-title": "Nat Rev Immunol",
"key": "B223",
"volume": "8",
"year": "2008"
},
{
"DOI": "10.3389/fimmu.2019.01296",
"article-title": "Conceptual approaches to modulating antibody effector functions and circulation half-life",
"author": "Saunders",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B224",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1006/cimm.2000.1617",
"article-title": "In vitro characterization of five humanized OKT3 effector function variant antibodies",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Cell Immunol",
"key": "B225",
"volume": "200",
"year": "2000"
},
{
"DOI": "10.1093/protein/gzw040",
"article-title": "Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions",
"author": "Schlothauer",
"doi-asserted-by": "publisher",
"journal-title": "Protein Eng Des Sel",
"key": "B226",
"volume": "29",
"year": "2016"
},
{
"DOI": "10.1172/JCI128437",
"article-title": "Antibodies targeting sialyl Lewis A mediate tumor clearance through distinct effector pathways",
"author": "Weitzenfeld",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Invest",
"key": "B227",
"volume": "129",
"year": "2019"
},
{
"DOI": "10.1038/s41577-022-00813-1",
"article-title": "Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection",
"author": "Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B228",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1371/journal.ppat.1011407",
"article-title": "Antibody-dependent cellular cytotoxicity, infected cell binding and neutralization by antibodies to the SIV envelope glycoprotein",
"author": "Grunst",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B229",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1128/JVI.00347-16",
"article-title": "Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 env-specific monoclonal antibodies",
"author": "von Bredow",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B230",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.1128/JVI.01808-20",
"article-title": "Relationships between neutralization, binding, and ADCC of broadly neutralizing antibodies against reservoir HIV",
"author": "Ren",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B231",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1038/ni.3158",
"article-title": "Antibody responses to envelope glycoproteins in HIV-1 infection",
"author": "Burton",
"doi-asserted-by": "publisher",
"journal-title": "Nat Immunol",
"key": "B232",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.3390/v13112236",
"article-title": "HIV-1 envelope glycoproteins proteolytic cleavage protects infected cells from ADCC mediated by plasma from infected individuals",
"author": "Prevost",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B233",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1128/mBio.00358-18",
"article-title": "Uninfected bystander cells impact the measurement of HIV-specific antibody-dependent cellular cytotoxicity responses",
"author": "Richard",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "B234",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1371/journal.ppat.1011569",
"article-title": "Non-neutralizing SARS-CoV-2 N-terminal domain antibodies protect mice against severe disease using Fc-mediated effector functions",
"author": "Pierre",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B235",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.1016/j.str.2020.03.001",
"article-title": "Antigen-induced allosteric changes in a human igG1 fc increase low-affinity fcgamma receptor binding",
"author": "Orlandi",
"doi-asserted-by": "publisher",
"first-page": "516",
"journal-title": "Structure",
"key": "B236",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1016/j.devcel.2014.04.031",
"article-title": "Actin cytoskeleton reorganization by Syk regulates Fcgamma receptor responsiveness by increasing its lateral mobility and clustering",
"author": "Jaumouille",
"doi-asserted-by": "publisher",
"journal-title": "Dev Cell",
"key": "B237",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.3389/fimmu.2020.01635",
"article-title": "Considerations of antibody geometric constraints on NK cell antibody dependent cellular cytotoxicity",
"author": "Murin",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B238",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1186/s12915-020-00819-y",
"article-title": "Defining rules governing recognition and Fc-mediated effector functions to the HIV-1 co-receptor binding site",
"author": "Tolbert",
"doi-asserted-by": "publisher",
"first-page": "91",
"journal-title": "BMC Biol",
"key": "B239",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1008083",
"article-title": "Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies",
"author": "Chu",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B240",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1038/s41467-018-04704-9",
"article-title": "A multifunctional human monoclonal neutralizing antibody that targets a unique conserved epitope on influenza HA",
"author": "Bangaru",
"doi-asserted-by": "publisher",
"first-page": "2669",
"journal-title": "Nat Commun",
"key": "B241",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0157788",
"article-title": "Enhancement of immune effector functions by modulating igG’s intrinsic affinity for target antigen",
"author": "Mazor",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B242",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.1056/NEJMoa0908492",
"article-title": "Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand",
"author": "Rerks-Ngarm",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B243",
"volume": "361",
"year": "2009"
},
{
"DOI": "10.1056/NEJMoa1113425",
"article-title": "Immune-correlates analysis of an HIV-1 vaccine efficacy trial",
"author": "Haynes",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B244",
"volume": "366",
"year": "2012"
},
{
"DOI": "10.1038/nature06106",
"article-title": "Fc receptor but not complement binding is important in antibody protection against HIV",
"author": "Hessell",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B245",
"volume": "449",
"year": "2007"
},
{
"DOI": "10.1038/nm.1974",
"article-title": "Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques",
"author": "Hessell",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B246",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.1126/scitranslmed.abn9662",
"article-title": "Antibody-mediated prevention of vaginal HIV transmission is dictated by IgG subclass in humanized mice",
"author": "Brady",
"doi-asserted-by": "publisher",
"journal-title": "Sci Transl Med",
"key": "B247",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1172/JCI122466",
"article-title": "Fc-dependent functions are redundant to efficacy of anti-HIV antibody PGT121 in macaques",
"author": "Parsons",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Invest",
"key": "B248",
"volume": "129",
"year": "2019"
},
{
"DOI": "10.1128/JVI.00491-12",
"article-title": "A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques",
"author": "Moldt",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B249",
"volume": "86",
"year": "2012"
},
{
"DOI": "10.1073/pnas.1103012108",
"article-title": "Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody",
"author": "Burton",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B250",
"volume": "108",
"year": "2011"
},
{
"DOI": "10.1016/j.ebiom.2016.11.024",
"article-title": "Neutralization takes precedence over igG or igA isotype-related functions in mucosal HIV-1 antibody-mediated protection",
"author": "Astronomo",
"doi-asserted-by": "publisher",
"first-page": "97",
"journal-title": "EBioMedicine",
"key": "B251",
"volume": "14",
"year": "2016"
},
{
"DOI": "10.1371/journal.ppat.1005042",
"article-title": "Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques",
"author": "Santra",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B252",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1038/s41577-021-00649-1",
"article-title": "Engaging innate immunity in HIV-1 cure strategies",
"author": "Board",
"doi-asserted-by": "publisher",
"first-page": "499",
"journal-title": "Nat Rev Immunol",
"key": "B253",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41467-025-56116-1",
"article-title": "Time to HIV viral rebound and frequency of post-treatment control after analytical interruption of antiretroviral therapy: an individual data-based meta-analysis of 24 prospective studies",
"author": "Gunst",
"doi-asserted-by": "publisher",
"first-page": "906",
"journal-title": "Nat Commun",
"key": "B254",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.1128/JVI.01911-15",
"article-title": "Envelope glycoprotein internalization protects human and simian immunodeficiency virus-infected cells from antibody-dependent cell-mediated cytotoxicity",
"author": "von Bredow",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B255",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.1128/JVI.00293-19",
"article-title": "Antibody-induced internalization of HIV-1 env proteins limits surface expression of the closed conformation of env",
"author": "Anand",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B256",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1073/pnas.1321507111",
"article-title": "Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity",
"author": "Arias",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B257",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1128/mbio.01827-24",
"article-title": "CD4 downregulation precedes Env expression and protects HIV-1-infected cells from ADCC mediated by non-neutralizing antibodies",
"author": "Richard",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "B258",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.celrep.2022.111624",
"article-title": "HIV-1 Vpu restricts Fc-mediated effector functions in vivo",
"author": "Prévost",
"doi-asserted-by": "publisher",
"first-page": "111624",
"journal-title": "Cell Rep",
"key": "B259",
"volume": "41",
"year": "2022"
},
{
"DOI": "10.1128/JVI.02779-15",
"article-title": "A highly conserved residue of the HIV-1 gp120 inner domain is important for antibody-dependent cellular cytotoxicity responses mediated by anti-cluster A antibodies",
"author": "Ding",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B260",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.1128/JVI.02868-14",
"article-title": "The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals",
"author": "Veillette",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B261",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.1086/605695",
"article-title": "HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection",
"author": "Santosuosso",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis",
"key": "B262",
"volume": "200",
"year": "2009"
},
{
"DOI": "10.1016/0042-6822(92)90593-E",
"article-title": "Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus",
"author": "Layne",
"doi-asserted-by": "publisher",
"first-page": "695",
"journal-title": "Virology",
"key": "B263",
"volume": "189",
"year": "1992"
},
{
"DOI": "10.1089/aid.2009.0290",
"article-title": "Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines",
"author": "Rychert",
"doi-asserted-by": "publisher",
"journal-title": "AIDS Res Hum Retroviruses",
"key": "B264",
"volume": "26",
"year": "2010"
},
{
"DOI": "10.1016/j.ebiom.2015.12.004",
"article-title": "Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity",
"author": "Richard",
"doi-asserted-by": "publisher",
"journal-title": "EBioMedicine",
"key": "B265",
"volume": "3",
"year": "2016"
},
{
"DOI": "10.1128/JVI.02194-14",
"article-title": "Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection",
"author": "Acharya",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B266",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1073/pnas.2222073120",
"article-title": "Indoline CD4-mimetic compounds mediate potent and broad HIV-1 inhibition and sensitization to antibody-dependent cellular cytotoxicity",
"author": "Fritschi",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A",
"key": "B267",
"volume": "120",
"year": "2023"
},
{
"DOI": "10.1128/jvi.00858-25",
"article-title": "CD4-mimetics sensitize HIV-infected cells to ADCC mediated by plasma from persons with early-stage HIV-1 infection",
"author": "Ding",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B268",
"volume": "99",
"year": "2025"
},
{
"DOI": "10.3390/v15051185",
"article-title": "Piperidine CD4-mimetic compounds expose vulnerable env epitopes sensitizing HIV-1-infected cells to ADCC",
"author": "Ding",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B269",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1038/s41467-021-21816-x",
"article-title": "Cryo-EM structures of HIV-1 trimer bound to CD4-mimetics BNM-III-170 and M48U1 adopt a CD4-bound open conformation",
"author": "Jette",
"doi-asserted-by": "publisher",
"first-page": "1950",
"journal-title": "Nat Commun",
"key": "B270",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.chom.2021.04.014",
"article-title": "Modulating HIV-1 envelope glycoprotein conformation to decrease the HIV-1 reservoir",
"author": "Rajashekar",
"doi-asserted-by": "publisher",
"first-page": "904",
"journal-title": "Cell Host Microbe",
"key": "B271",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1038/s41467-018-04758-9",
"article-title": "A CD4-mimetic compound enhances vaccine efficacy against stringent immunodeficiency virus challenge",
"author": "Madani",
"doi-asserted-by": "publisher",
"first-page": "2363",
"journal-title": "Nat Commun",
"key": "B272",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.xcrm.2022.100510",
"article-title": "SARS-CoV-2 Beta and Delta variants trigger Fc effector function with increased cross-reactivity",
"author": "Richardson",
"doi-asserted-by": "publisher",
"first-page": "100510",
"journal-title": "Cell Rep Med",
"key": "B273",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2021.06.001",
"article-title": "A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses",
"author": "Tauzin",
"doi-asserted-by": "publisher",
"first-page": "1137",
"journal-title": "Cell Host Microbe",
"key": "B274",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/j.immuni.2022.01.001",
"article-title": "mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern",
"author": "Kaplonek",
"doi-asserted-by": "publisher",
"first-page": "355",
"journal-title": "Immunity",
"key": "B275",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1038/s41392-021-00759-1",
"article-title": "Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "346",
"journal-title": "Signal Transduct Target Ther",
"key": "B276",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.xcrm.2021.100296",
"article-title": "Decay of Fc-dependent antibody functions after mild to moderate COVID-19",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "100296",
"journal-title": "Cell Rep Med",
"key": "B277",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1016/j.xcrm.2022.100893",
"article-title": "The Fc-effector function of COVID-19 convalescent plasma contributes to SARS-CoV-2 treatment efficacy in mice",
"author": "Ullah",
"doi-asserted-by": "publisher",
"first-page": "100893",
"journal-title": "Cell Rep Med",
"key": "B278",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2021.06.005",
"article-title": "SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2",
"author": "Amanat",
"doi-asserted-by": "publisher",
"first-page": "3936",
"journal-title": "Cell",
"key": "B279",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and safety of the mRNA-1273 SARS-coV-2 vaccine",
"author": "Baden",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B280",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine",
"author": "Polack",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B281",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.immuni.2021.08.015",
"article-title": "Live imaging of SARS-CoV-2 infection in mice reveals that neutralizing antibodies require Fc function for optimal efficacy",
"author": "Ullah",
"doi-asserted-by": "publisher",
"first-page": "2143",
"journal-title": "Immunity",
"key": "B282",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.02.026",
"article-title": "Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection",
"author": "Winkler",
"doi-asserted-by": "publisher",
"first-page": "1804",
"journal-title": "Cell",
"key": "B283",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1038/s41564-023-01359-1",
"article-title": "Fc-gammaR-dependent antibody effector functions are required for vaccine-mediated protection against antigen-shifted variants of SARS-CoV-2",
"author": "Mackin",
"doi-asserted-by": "publisher",
"journal-title": "Nat Microbiol",
"key": "B284",
"volume": "8",
"year": "2023"
}
],
"reference-count": 284,
"references-count": 284,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2025.1733684/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Viral glycoprotein-mediated entry and antibody-mediated immunity in HIV-1 and SARS-CoV-2 infection",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "16"
}