Effect of Adding Losartan to Standard of Care Treatment on the Risk of Death and Icu Admission Among Hospitalized COVID-19 Patients: A Randomized Trial
et al., SSRN Electronic Journal, doi:10.2139/ssrn.4278529, Dec 2022
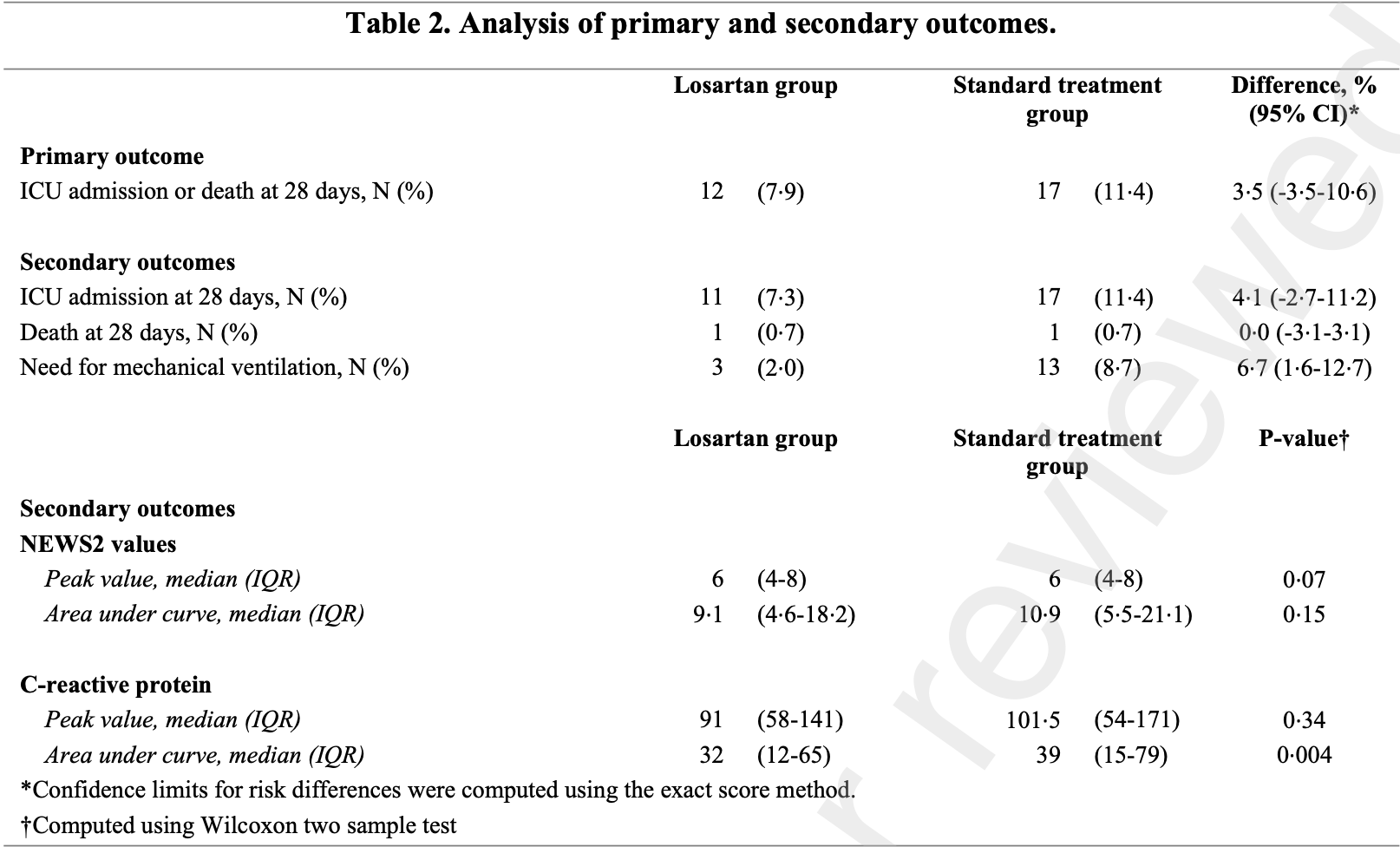
RCT 302 hospitalized COVID-19 patients showing lower mechanical ventilation but no significant difference in ICU admission or mortality with losartan treatment.
|
risk of death, 1.3% lower, RR 0.99, p = 1.00, treatment 1 of 151 (0.7%), control 1 of 149 (0.7%), NNT 11250.
|
|
risk of mechanical ventilation, 77.2% lower, RR 0.23, p = 0.01, treatment 3 of 151 (2.0%), control 13 of 149 (8.7%), NNT 15.
|
|
risk of ICU admission, 36.2% lower, RR 0.64, p = 0.24, treatment 11 of 151 (7.3%), control 17 of 149 (11.4%), NNT 24.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Götberg et al., 31 Dec 2022, Randomized Controlled Trial, Sweden, peer-reviewed, 11 authors.
EFFECT OF ADDING LOSARTAN TO STANDARD OF CARE TREATMENT ON THE RISK OF DEATH AND ICU ADMISSION AMONG HOSPITALIZED COVID-19 PATIENTS: A RANDOMIZED TRIAL
Background: Angiotensin-converting enzyme 2 (ACE2) is the host receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Recognizing that angiotensin receptor blockers upregulate the expression of ACE2, it has been proposed that these drugs may have a beneficial effect in Covid-19.
Methods: We did an open-label randomized controlled trial to test whether addition of losartan to standard of care lowers the risk of death or intensive care unit (ICU) admission in patients with confirmed SARS-CoV-2 infection. The trial was run in a secondary hospital in Stockholm, Sweden between October 5th 2020 and June 21st 2021. Included patients were randomized using a 1:1 allocation ratio, to receive standard care or losartan in addition to standard care, using an electronic tool. The study was not blinded. The primary outcome was a composite of ICU admission or death within 28 days of admission.
Findings: The study was terminated for futility after a planned interim analysis. A total of 302 patients were included of whom 151 were assigned to each group. The primary outcome occurred in 12 (7•9%) in the intervention group and 17 (11•4%) in the standard-of-care group (risk difference, 3•5 percentage points; 95% confidence interval, -3•5-11). Among secondary outcomes, there were no differences between the groups in the occurrence of death (p=1•00), ICU admission (p=0•24), or the National Early Warning Scale 2 (NEWS2) score levels (p=0•15), but patients randomized to losartan treatment had a 6•7 percentage points lower risk of mechanical ventilation (95% confidence interval, 1•6-12•7) and 12•5 mg/L lower c-reactive protein concentration (95% confidence interval, 2•8-22 mg/L; p=0•01). Interpretation: Addition of losartan to standard care treatment did not result in a lower occurrence of the primary outcome of death or ICU admission.
CONTRIBUTORS All authors participated in the conceptualization and planning of the study. GE, MC, MR, and AH wrote the study protocol. All authors participated in the enrolment of patients. AG, GE, RN and AH collected the data. GE performed the statistical analyses. AG wrote the first draft of the manuscript together with GE and AH. All authors contributed to the review and editing of the manuscript.
DECLARATION OF INTERESTS All authors declare that they have no conflicts of interest. This preprint research paper has not been peer reviewed. Electronic copy available at: https://ssrn.com/abstract=4278529 P r e p r i n t n o t p e e r r e v i e w e d This preprint research paper has not been peer reviewed. Electronic copy available at: https://ssrn.com/abstract=4278529 P r e p r i n t n o t p e e r r e v i e w e d This preprint research paper has not been peer reviewed. Electronic copy available at: https://ssrn.com/abstract=4278529 P r e p r i n t n o t p e e r r e v i e w e d
TABLES
Troponin
References
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Cheng, Wang, Wang, Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19, J Med Virol
Ciulla, SARS-CoV-2 downregulation of ACE2 and pleiotropic effects of ACEIs/ARBs, Hypertens Res
Dougan, Nirula, Azizad, Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med
Duarte, Pelorosso, Nicolosi, Telmisartan for treatment of Covid-19 patients: An open multicenter randomized clinical trial, EClinicalMedicine
Geriak, Haddad, Kullar, Randomized Prospective Open Label Study Shows No Impact on Clinical Outcome of Adding Losartan to Hospitalized COVID-19 Patients with Mild Hypoxemia, Infect Dis Ther
Grasselli, Zangrillo, Zanella, Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy, JAMA
Gray, A Class of $K$-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk, The Annals of Statistics
Group, Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Guo, Huang, Lin, Lv, Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection, J Am Heart Assoc
Hamming, Timens, Bulthuis, Lely, Navis et al., Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J Pathol
Harris, Taylor, Minor, The REDCap consortium: Building an international community of software platform partners, J Biomed Inform
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell
Lopes, Macedo, De Barros, Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial, JAMA
Miettinen, Nurminen, Comparative analysis of two rates, Stat Med
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med
Puskarich, Cummins, Ingraham, A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19, EClinicalMedicine
Puskarich, Ingraham, Merck, Efficacy of Losartan in Hospitalized Patients With COVID-19-Induced Lung Injury: A Randomized Clinical Trial, JAMA Netw Open
Rosas, Brau, Waters, Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia, N Engl J Med
Sadoff, Gray, Vandebosch, Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19, N Engl J Med
Voysey, Clemens, Madhi, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Wan, Shang, Graham, Baric, Li, Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus, J Virol
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N Engl J Med
Yang, Yu, Xu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med
Zhang, Luo, Li, Risk Factors for Death Among the First 80 543 COVID-19 Cases in China: Relationships Between Age, Underlying Disease, Case Severity, and Region, Clin Infect Dis
Zhang, Zhu, Cai, Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19, Circ Res
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
Zhu, Zhang, Wang, A Novel Coronavirus from Patients with Pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.2139/ssrn.4278529",
"ISSN": [
"1556-5068"
],
"URL": "http://dx.doi.org/10.2139/ssrn.4278529",
"author": [
{
"affiliation": [],
"family": "Götberg",
"given": "Alice",
"sequence": "first"
},
{
"affiliation": [],
"family": "Edgren",
"given": "Gustaf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouleau",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hollenberg",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ringh",
"given": "Mattias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sundelin",
"given": "Runa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Treutiger",
"given": "Carl Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nyström",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cronhjort",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hedman",
"given": "Anders",
"sequence": "additional"
}
],
"container-title": "SSRN Electronic Journal",
"container-title-short": "SSRN Journal",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
20
]
],
"date-time": "2022-11-20T03:08:35Z",
"timestamp": 1668913715000
},
"deposited": {
"date-parts": [
[
2022,
11,
20
]
],
"date-time": "2022-11-20T03:08:51Z",
"timestamp": 1668913731000
},
"indexed": {
"date-parts": [
[
2022,
11,
21
]
],
"date-time": "2022-11-21T05:32:12Z",
"timestamp": 1669008732813
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022
]
]
},
"language": "en",
"member": "78",
"original-title": [],
"prefix": "10.2139",
"published": {
"date-parts": [
[
2022
]
]
},
"published-other": {
"date-parts": [
[
2022
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ssrn.com/abstract=4278529"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of Adding Losartan to Standard of Care Treatment on the Risk of Death and Icu Admission Among Hospitalized COVID-19 Patients: A Randomized Trial",
"type": "journal-article"
}