Phase 2 study of oral sabizabulin for the treatment of SARS-CoV-2 in hospitalized patients at high risk for ARDS
, M., European Congress of Clinical Microbiology & Infectious Diseases, Apr 2022
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
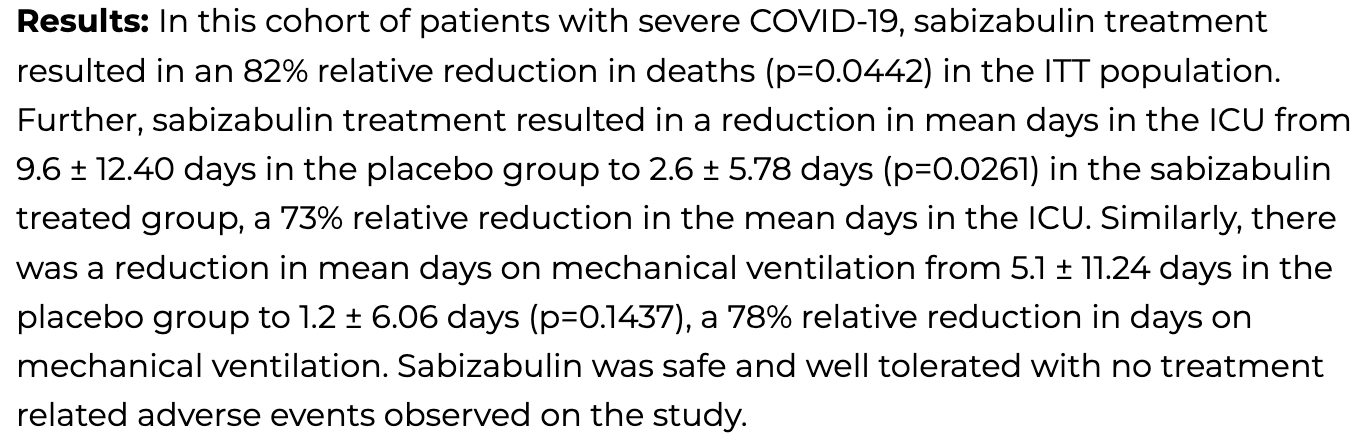
Phase 2 RCT of sabizabulin showing lower mortality with treatment. For more discussion see1.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 82.0% lower, RR 0.18, p = 0.04, ITT.
|
|
ventilation time, 76.5% lower, relative time 0.24, p = 0.14.
|
|
ICU time, 72.9% lower, relative time 0.27, p = 0.03.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gordon et al., 25 Apr 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 1 author.
