Efficacy and safety of combined nebulization of unfractionated heparin, acetylcysteine, budesonide and ipratropium bromide in hospitalised patients with COVID-19 pneumonia: a randomized controlled clinical trial
et al., BMC Pulmonary Medicine, doi:10.1186/s12890-025-03824-5, ChiCTR2300073871, Jul 2025
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
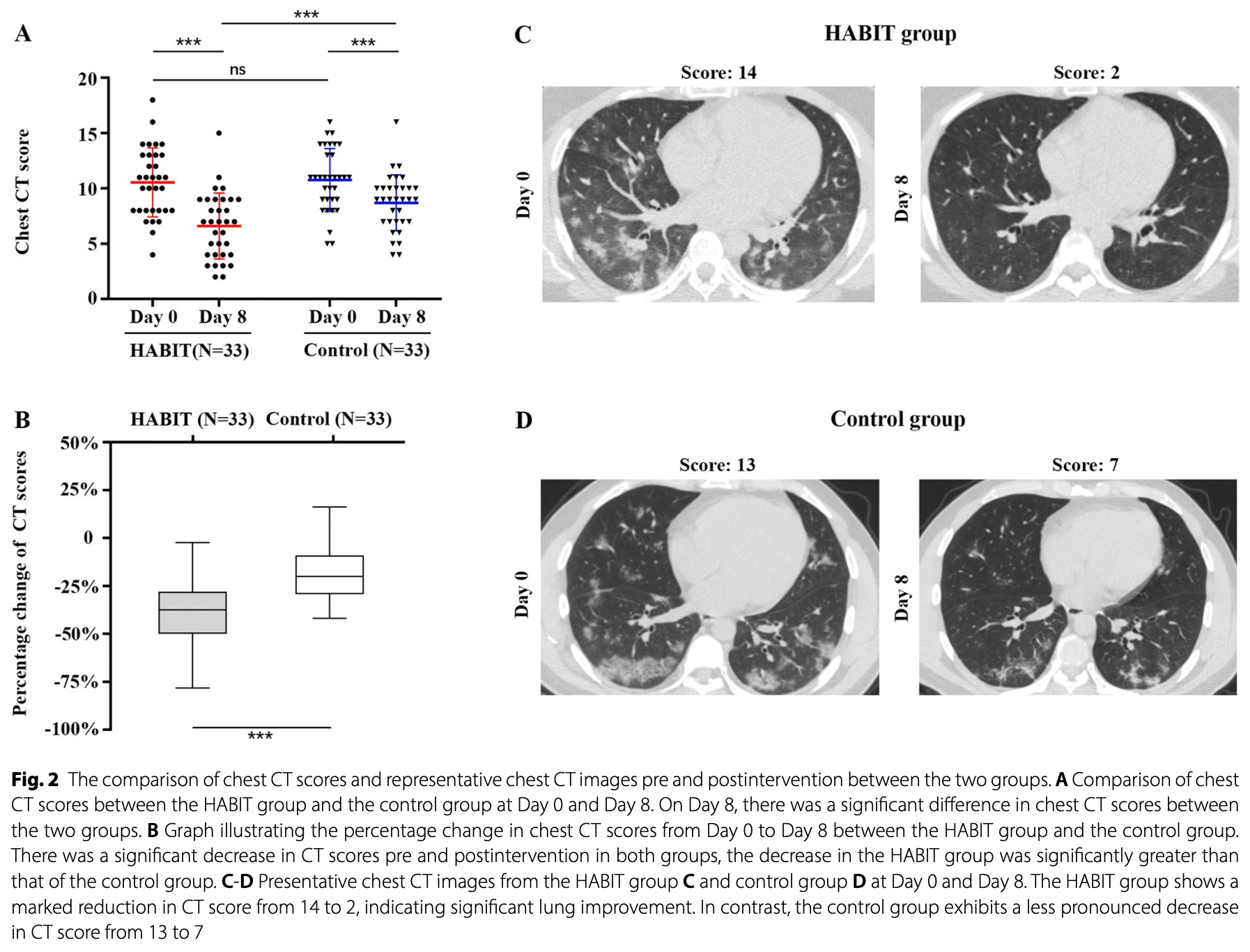
RCT 74 hospitalized COVID-19 pneumonia patients showing improved lung lesion absorption and oxygenation with combined nebulization of unfractionated heparin, acetylcysteine, budesonide, and ipratropium bromide. The treatment group demonstrated significantly lower CT scores post-treatment (6.6 vs 8.69, p=0.003) and greater lesion absorption (37.5% vs 20%, p<0.001) compared to standard care. More patients in the treatment group achieved PaO₂/FiO₂ ≥400 mmHg (32.3% vs 6.5%, p=0.022) and had lower CRP levels (1.69 vs 3.52, p=0.03) post-treatment. 76% of control patients also received nebulization with one or more components of the treatment, including 70% that received acetylcysteine and budesonide.
Study covers budesonide and N-acetylcysteine.
Gong et al., 22 Jul 2025, Randomized Controlled Trial, China, peer-reviewed, 18 authors, this trial uses multiple treatments in the treatment arm (combined with heparin, acetylcysteine, budesonide, ipratropium bromide) - results of individual treatments may vary, trial ChiCTR2300073871.
Contact: li676@sina.com, cgq1963@tmmu.edu.cn, dpyyhxlili@tmmu.edu.cn.
Efficacy and safety of combined nebulization of unfractionated heparin, acetylcysteine, budesonide and ipratropium bromide in hospitalised patients with COVID-19 pneumonia: a randomized controlled clinical trial
BMC Pulmonary Medicine, doi:10.1186/s12890-025-03824-5
Background Promoting the absorption of COVID-19 pneumonia is critical for reducing pulmonary sequelae and improving prognosis. This study aimed to evaluate the efficacy and safety of nebulized unfractionated heparin, acetylcysteine, budesonide, and ipratropium bromide (HABIT) in hospitalized patients with COVID-19 pneumonia. Methods This single-center, open-label, randomized, parallel-group trial was conducted at a tertiary hospital in China. Participants were randomized 1:1 to receive either standard of care (SOC) or SOC plus nebulized HABIT. The HABIT protocol included daily quadruple nebulization for seven days, comprising 6000 units heparin sodium, 2 mg budesonide, 0.3 g acetylcysteine, and 0.5 mg ipratropium bromide. The primary outcome was the change in lung lesions assessed by chest CT scans on admission (Day 0) and post-treatment (Day 8). Results A total of 74 patients were randomized to the HABIT group (n = 37) or the control group (n = 37). Four patients per group were excluded during follow-up, leaving 66 patients for final analysis. Baseline CT scores were comparable between groups (10.55 ± 3.11 vs. 10.76 ± 2.85, p = 0.774). Post-treatment, the HABIT group showed significantly lower mean CT scores (6.6 ± 2.98 vs. 8.69 ± 2.53, p = 0.003) and greater lesion absorption (37.5% vs. 20%,
Abbreviations
Consent for publication Not Applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Agustí, Stefano, Levi, Add-on inhaled Budesonide in the treatment of hospitalised patients with COVID-19: a randomised clinical trial, Eur Respir J
Cazzola, Ora, Bianco, Rogliani, Matera, Guidance on nebulization during the current COVID-19 pandemic, Respir Med
Chen, Zhu, Zheng, Zhao, Liu, Clinical efficacy of Budesonide combined with acetylcysteine in the treatment of mycoplasma pneumonia infection, Immun Inflamm Dis
Da, Grillo, Bertanha, Da, Rodrigues, Nebulized enriched heparin improves respiratory parameters in patients with COVID-19: a phase I/II randomized and triple-blind clinical trial, Sci Rep
Denucci, Wilkinson, Sverdloff, Inhaled nebulised unfractionated heparin (UFH) for the treatment of hospitalised patients with COVID-19: A randomised controlled pilot study, Pulm Pharmacol Ther
Elsharnouby, Eid, Elezz, Aboelatta, Heparin/N-acetylcysteine: an adjuvant in the management of burn inhalation injury, J Crit Care
Enkhbaatar, Pruitt, Suman, Pathophysiology, research challenges, and clinical management of smoke inhalation injury, Lancet
Erelel, Kaskal, Akbal-Dagistan, Early effects of low molecular weight heparin therapy with Soft-Mist inhaler for COVID-19-Induced hypoxemia: A phase IIb trial, Pharmaceutics
Francone, Iafrate, Masci, Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis, Eur Radiol
Grasselli, Tonetti, Protti, Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study, Lancet Respir Med
Gupta, Ahluwalia, Gupta, Gupta, Role of nebulized heparin in clinical outcome of COVID-19 patients with respiratory symptoms: A systematic review, Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med
Gupta, Chandrakar, Gupta, Jain, Nebulized heparin to reduce COVID-19-induced acute lung injury: A prospective observational study, Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med
Han, Chen, Guo, Long-term radiological and pulmonary function abnormalities at 3 years after COVID-19 hospitalisation: a longitudinal cohort study, Eur Respir J
Huang, Li, Gu, Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study, Lancet Respir Med
Izquierdo-Alonso, Pérez-Rial, Rivera, Peces-Barba, N-acetylcysteine for prevention and treatment of COVID-19: current state of evidence and future directions, J Infect Public Health
Jones, Williams, Cairns, Cartotto, INHALATION INJURY: pathophysiology, diagnosis, and treatment, Clin Plast Surg
Lana, Lana, Rodrigues, Nebulization of glutathione and N-Acetylcysteine as an adjuvant therapy for COVID-19 onset, Adv Redox Res
Moustafa, Elbery, Meslamani, Okda, Alsfouk et al., Evaluating the use of inhaled Budesonide and Ipratropium bromide combination in patients at high risk of acute respiratory distress syndrome development: A randomized controlled trial, Pharmaceuticals
Nigro, Valenzuela, Arancibia, A worldwide look into long COVID-19 management: an END-COVID survey, ERJ Open Res
Niknam, Jafari, Golchin, Potential therapeutic options for COVID-19: an update on current evidence, Eur J Med Res
O'reilly, Pulmonary fibrosis in COVID-19: mechanisms, consequences and targets, QJM
Pan, Ye, Sun, Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19), Radiology
Panahi, Ghanei, Rahimi, Evaluation the efficacy and safety of N-acetylcysteine inhalation spray in controlling the symptoms of patients with COVID-19: an open-label randomized controlled clinical trial, J Med Virol
Ramakrishnan, Nicolau, Langford, Inhaled Budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med
Saha, Quiñones-Mateu, Das, Inhaled therapy for COVID-19: considerations of drugs, formulations and devices, Int J Pharm
Selickman, Vrettou, Mentzelopoulos, Marini, COVID-19-Related ARDS: key mechanistic features and treatments, J Clin Med
Sheridan, Ingelfinger, Fire-related inhalation injury, N Engl J Med
The, Heart, Randomized, Placebo-controlled clinical trial of an aerosolized β2-Agonist for treatment of acute lung injury, Am J Respir Crit Care Med
Van Haren, Page, Laffey, Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence, Crit Care Lond Engl
Van Haren, Van Loon, Steins, Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: A multicentre case series of 98 patients, Br J Clin Pharmacol
Villamañán, Sobrino, Carpio, Inhaled bronchodilators use and clinical course of adult inpatients with Covid-19 pneumonia in spain: A retrospective cohort study, Pulm Pharmacol Ther
Vreeman, Pillay, Burgess, Post-COVID pulmonary sequelae: mechanisms and potential targets to reduce persistent fibrosis, Pharmacol Ther
Wang, Dong, Hu, Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study, Radiology Published Online
Wang, Liu, Chen, Zhang, Acetylcysteine and Budesonide for the treatment of refractory Mycoplasma pneumoniae pneumonia in children: a clinical observation, Ital J Pediatr
Wang, Zhang, Qian, Liu, Zhang, Budesonide combined with Ipratropium bromide in the treatment of Bronchopneumonia and its efficacy on pulmonary function, Int J Clin Exp Med
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review, JAMA
Wu, Chen, Cai, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China, JAMA Intern Med
Yu, Bafadhel, Dorward, Inhaled Budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet Lond Engl
Yu, Choi, Ryoo, Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease 2019: A living review and meta-analysis, PLoS ONE
Yuan, Jiao, Qu, Yang, Liu, The development of COVID-19 treatment, Front Immunol
Yun, Wang, Hao, Xu, Cai, The time course of chest CT lung changes in COVID-19 patients from onset to discharge, Eur J Radiol Open
Zhang, Huang, Gu, 3-year outcomes of discharged survivors of COVID-19 following the SARS-CoV-2 Omicron (B.1.1.529) wave in 2022 in china: a longitudinal cohort study, Lancet Respir Med
Zhao, Ma, Yue, Chen, SARS-CoV-2 infection and lung regeneration, Clin Microbiol Rev
Zu, Jiang, Xu, Coronavirus disease 2019 (COVID-19): A perspective from China, Radiology
Çelik İ Öztürkr, From asymptomatic to critical illness: decoding various clinical stages of COVID-19, Turk J Med Sci
DOI record:
{
"DOI": "10.1186/s12890-025-03824-5",
"ISSN": [
"1471-2466"
],
"URL": "http://dx.doi.org/10.1186/s12890-025-03824-5",
"alternative-id": [
"3824"
],
"article-number": "346",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "15 May 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "30 June 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "22 July 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was approved by the Ethics Committee of Daping Hospital, Army Medical University (NO.2023-137-01). All patients provided written informed consent. The study was conducted and reported following the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not Applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Gong",
"given": "Junhui",
"sequence": "first"
},
{
"affiliation": [],
"family": "Nie",
"given": "Naifu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Minrui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Xinyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Qinghua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deng",
"given": "Jia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kang",
"given": "Jun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Nuo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du",
"given": "Xiaoyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Hui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Kunlin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Guoqiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Li",
"sequence": "additional"
}
],
"container-title": "BMC Pulmonary Medicine",
"container-title-short": "BMC Pulm Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
7,
22
]
],
"date-time": "2025-07-22T09:38:59Z",
"timestamp": 1753177139000
},
"deposited": {
"date-parts": [
[
2025,
7,
22
]
],
"date-time": "2025-07-22T09:39:10Z",
"timestamp": 1753177150000
},
"funder": [
{
"award": [
"CSTB2023NSCQ-JQX0033"
],
"name": "the Outstanding Youth Science Fund of Chongqing Municipal Science and Technology Bureau"
},
{
"DOI": "10.13039/100016834",
"award": [
"2023jstg012"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100016834",
"id-type": "DOI"
}
],
"name": "Chongqing Municipal Health Commission"
},
{
"DOI": "10.13039/501100012397",
"award": [
"2022XL004, ZXAIZD001"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100012397",
"id-type": "DOI"
}
],
"name": "Army Medical University"
}
],
"indexed": {
"date-parts": [
[
2025,
7,
22
]
],
"date-time": "2025-07-22T10:10:14Z",
"timestamp": 1753179014336,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
7,
22
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
22
]
],
"date-time": "2025-07-22T00:00:00Z",
"timestamp": 1753142400000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
22
]
],
"date-time": "2025-07-22T00:00:00Z",
"timestamp": 1753142400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12890-025-03824-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12890-025-03824-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12890-025-03824-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
7,
22
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
22
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1183/23120541.00096-2024",
"doi-asserted-by": "crossref",
"key": "3824_CR1",
"unstructured": "Nigro M, Valenzuela C, Arancibia F, et al. A worldwide look into long COVID-19 management: an END-COVID survey. ERJ Open Res. 2024;10(6):00096-2024."
},
{
"author": "ÖZTÜRKR ÇELİK İ",
"first-page": "3284",
"issue": "7",
"journal-title": "Turk J Med Sci",
"key": "3824_CR2",
"unstructured": "ÇELİK İ ÖZTÜRKR. From asymptomatic to critical illness: decoding various clinical stages of COVID-19. Turk J Med Sci. 2021;51(7):3284–300.",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30370-2",
"author": "G Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1201",
"issue": "12",
"journal-title": "Lancet Respir Med",
"key": "3824_CR3",
"unstructured": "Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8(12):1201–8.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/jcm11164896",
"author": "J Selickman",
"doi-asserted-by": "crossref",
"first-page": "4896",
"issue": "16",
"journal-title": "J Clin Med",
"key": "3824_CR4",
"unstructured": "Selickman J, Vrettou CS, Mentzelopoulos SD, Marini JJ. COVID-19-Related ARDS: key mechanistic features and treatments. J Clin Med. 2022;11(16):4896.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"author": "C Wu",
"doi-asserted-by": "crossref",
"first-page": "934",
"issue": "7",
"journal-title": "JAMA Intern Med",
"key": "3824_CR5",
"unstructured": "Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020;180(7):934–43.",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(22)00126-6",
"author": "L Huang",
"doi-asserted-by": "crossref",
"first-page": "863",
"issue": "9",
"journal-title": "Lancet Respir Med",
"key": "3824_CR6",
"unstructured": "Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10(9):863–76.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(23)00387-9",
"author": "H Zhang",
"doi-asserted-by": "crossref",
"first-page": "55",
"issue": "1",
"journal-title": "Lancet Respir Med",
"key": "3824_CR7",
"unstructured": "Zhang H, Huang C, Gu X, et al. 3-year outcomes of discharged survivors of COVID-19 following the SARS-CoV-2 Omicron (B.1.1.529) wave in 2022 in china: a longitudinal cohort study. Lancet Respir Med. 2024;12(1):55–66.",
"volume": "12",
"year": "2024"
},
{
"DOI": "10.1128/cmr.00188-21",
"author": "F Zhao",
"doi-asserted-by": "crossref",
"first-page": "e00188",
"issue": "2",
"journal-title": "Clin Microbiol Rev",
"key": "3824_CR8",
"unstructured": "Zhao F, Ma Q, Yue Q, Chen H. SARS-CoV-2 infection and lung regeneration. Clin Microbiol Rev. 2022;35(2):e00188.",
"volume": "35",
"year": "2022"
},
{
"DOI": "10.1183/13993003.01612-2023",
"doi-asserted-by": "crossref",
"key": "3824_CR9",
"unstructured": "Han X, Chen L, Guo L, et al. Long-term radiological and pulmonary function abnormalities at 3 years after COVID-19 hospitalisation: a longitudinal cohort study. Eur Respir J. 2024;64(1):2301612."
},
{
"DOI": "10.1186/s40001-021-00626-3",
"author": "Z Niknam",
"doi-asserted-by": "crossref",
"first-page": "6",
"issue": "1",
"journal-title": "Eur J Med Res",
"key": "3824_CR10",
"unstructured": "Niknam Z, Jafari A, Golchin A, et al. Potential therapeutic options for COVID-19: an update on current evidence. Eur J Med Res. 2022;27(1):6.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2023.1125246",
"author": "Y Yuan",
"doi-asserted-by": "crossref",
"first-page": "1125246",
"journal-title": "Front Immunol",
"key": "3824_CR11",
"unstructured": "Yuan Y, Jiao B, Qu L, Yang D, Liu R. The development of COVID-19 treatment. Front Immunol. 2023;14:1125246.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.ijpharm.2022.122042",
"author": "T Saha",
"doi-asserted-by": "crossref",
"first-page": "122042",
"journal-title": "Int J Pharm",
"key": "3824_CR12",
"unstructured": "Saha T, Quiñones-Mateu ME, Das SC. Inhaled therapy for COVID-19: considerations of drugs, formulations and devices. Int J Pharm. 2022;624:122042.",
"volume": "624",
"year": "2022"
},
{
"DOI": "10.1016/j.rmed.2020.106236",
"author": "M Cazzola",
"doi-asserted-by": "crossref",
"first-page": "106236",
"journal-title": "Respir Med",
"key": "3824_CR13",
"unstructured": "Cazzola M, Ora J, Bianco A, Rogliani P, Matera MG. Guidance on nebulization during the current COVID-19 pandemic. Respir Med. 2021;176:106236.",
"volume": "176",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.12839",
"author": "WJ Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"issue": "8",
"journal-title": "JAMA",
"key": "3824_CR14",
"unstructured": "Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324(8):782–93.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03148-2",
"author": "FMP van Haren",
"doi-asserted-by": "crossref",
"first-page": "454",
"issue": "1",
"journal-title": "Crit Care Lond Engl",
"key": "3824_CR15",
"unstructured": "van Haren FMP, Page C, Laffey JG, et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit Care Lond Engl. 2020;24(1):454.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.3390/pharmaceutics13111768",
"author": "M Erelel",
"doi-asserted-by": "crossref",
"first-page": "1768",
"issue": "11",
"journal-title": "Pharmaceutics",
"key": "3824_CR16",
"unstructured": "Erelel M, Kaskal M, Akbal-Dagistan O, et al. Early effects of low molecular weight heparin therapy with Soft-Mist inhaler for COVID-19-Induced hypoxemia: A phase IIb trial. Pharmaceutics. 2021;13(11):1768.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.pupt.2023.102212",
"author": "G DeNucci",
"doi-asserted-by": "crossref",
"first-page": "102212",
"journal-title": "Pulm Pharmacol Ther",
"key": "3824_CR17",
"unstructured": "DeNucci G, Wilkinson T, Sverdloff C, et al. Inhaled nebulised unfractionated heparin (UFH) for the treatment of hospitalised patients with COVID-19: A randomised controlled pilot study. Pulm Pharmacol Ther. 2023;80:102212.",
"volume": "80",
"year": "2023"
},
{
"DOI": "10.1111/bcp.15212",
"author": "FMP van Haren",
"doi-asserted-by": "crossref",
"first-page": "2802",
"issue": "6",
"journal-title": "Br J Clin Pharmacol",
"key": "3824_CR18",
"unstructured": "van Haren FMP, van Loon LM, Steins A, et al. Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: A multicentre case series of 98 patients. Br J Clin Pharmacol. 2022;88(6):2802–13.",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1038/s41598-024-70064-8",
"author": "VT Ramos da Silva Grillo",
"doi-asserted-by": "crossref",
"first-page": "19902",
"issue": "1",
"journal-title": "Sci Rep",
"key": "3824_CR19",
"unstructured": "Ramos da Silva Grillo VT, Bertanha M, da Silva Rodrigues L, et al. Nebulized enriched heparin improves respiratory parameters in patients with COVID-19: a phase I/II randomized and triple-blind clinical trial. Sci Rep. 2024;14(1):19902.",
"volume": "14",
"year": "2024"
},
{
"author": "B Gupta",
"first-page": "572",
"issue": "8",
"journal-title": "Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med",
"key": "3824_CR20",
"unstructured": "Gupta B, Ahluwalia P, Gupta N, Gupta A. Role of nebulized heparin in clinical outcome of COVID-19 patients with respiratory symptoms: A systematic review. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2023;27(8):572–9.",
"volume": "27",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28393",
"author": "Y Panahi",
"doi-asserted-by": "crossref",
"first-page": "e28393",
"issue": "1",
"journal-title": "J Med Virol",
"key": "3824_CR21",
"unstructured": "Panahi Y, Ghanei M, Rahimi M, et al. Evaluation the efficacy and safety of N-acetylcysteine inhalation spray in controlling the symptoms of patients with COVID‐19: an open‐label randomized controlled clinical trial. J Med Virol. 2023;95(1):e28393.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.jiph.2022.11.009",
"author": "JL Izquierdo-Alonso",
"doi-asserted-by": "crossref",
"first-page": "1477",
"issue": "12",
"journal-title": "J Infect Public Health",
"key": "3824_CR22",
"unstructured": "Izquierdo-Alonso JL, Pérez-Rial S, Rivera CG, Peces-Barba G. N-acetylcysteine for prevention and treatment of COVID-19: current state of evidence and future directions. J Infect Public Health. 2022;15(12):1477–83.",
"volume": "15",
"year": "2022"
},
{
"author": "N-acetylcysteine for",
"first-page": "94",
"issue": "1",
"journal-title": "J Infect",
"key": "3824_CR23",
"unstructured": "N-acetylcysteine for. The treatment of COVID-19 among hospitalized patients. J Infect. 2022;84(1):94–118.",
"volume": "84",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"author": "S Ramakrishnan",
"doi-asserted-by": "crossref",
"first-page": "763",
"issue": "7",
"journal-title": "Lancet Respir Med",
"key": "3824_CR24",
"unstructured": "Ramakrishnan S, Nicolau DV, Langford B, et al. Inhaled Budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9(7):763–72.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"author": "LM Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"issue": "10303",
"journal-title": "Lancet Lond Engl",
"key": "3824_CR25",
"unstructured": "Yu LM, Bafadhel M, Dorward J, et al. Inhaled Budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Lond Engl. 2021;398(10303):843–55.",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1183/13993003.03036-2021",
"author": "A Agustí",
"doi-asserted-by": "crossref",
"first-page": "2103036",
"issue": "3",
"journal-title": "Eur Respir J",
"key": "3824_CR26",
"unstructured": "Agustí A, De Stefano G, Levi A, et al. Add-on inhaled Budesonide in the treatment of hospitalised patients with COVID-19: a randomised clinical trial. Eur Respir J. 2022;59(3):2103036.",
"volume": "59",
"year": "2022"
},
{
"DOI": "10.1164/rccm.201012-2090OC",
"author": "L Randomized",
"doi-asserted-by": "crossref",
"first-page": "561",
"issue": "5",
"journal-title": "Am J Respir Crit Care Med",
"key": "3824_CR27",
"unstructured": "The National Heart, Randomized L. Placebo-controlled clinical trial of an aerosolized β2-Agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561.",
"volume": "184",
"year": "2011"
},
{
"DOI": "10.1016/j.pupt.2021.102007",
"author": "E Villamañán",
"doi-asserted-by": "crossref",
"first-page": "102007",
"journal-title": "Pulm Pharmacol Ther",
"key": "3824_CR28",
"unstructured": "Villamañán E, Sobrino C, Carpio C, et al. Inhaled bronchodilators use and clinical course of adult inpatients with Covid-19 pneumonia in spain: A retrospective cohort study. Pulm Pharmacol Ther. 2021;69:102007.",
"volume": "69",
"year": "2021"
},
{
"DOI": "10.3390/ph18030412",
"author": "HAM Moustafa",
"doi-asserted-by": "crossref",
"first-page": "412",
"issue": "3",
"journal-title": "Pharmaceuticals",
"key": "3824_CR29",
"unstructured": "Moustafa HAM, Elbery FH, Al Meslamani AZ, Okda SM, Alsfouk BA, Kassem AB. Evaluating the use of inhaled Budesonide and Ipratropium bromide combination in patients at high risk of acute respiratory distress syndrome development: A randomized controlled trial. Pharmaceuticals. 2025;18(3):412.",
"volume": "18",
"year": "2025"
},
{
"DOI": "10.1056/NEJMra1601128",
"author": "RL Sheridan",
"doi-asserted-by": "crossref",
"first-page": "464",
"issue": "5",
"journal-title": "N Engl J Med.",
"key": "3824_CR30",
"unstructured": "Sheridan RL. Fire-related inhalation injury. Ingelfinger JR, ed. N Engl J Med. 2016;375(5):464–9.",
"volume": "375",
"year": "2016"
},
{
"DOI": "10.1016/S0140-6736(16)31458-1",
"author": "P Enkhbaatar",
"doi-asserted-by": "crossref",
"first-page": "1437",
"issue": "10052",
"journal-title": "Lancet",
"key": "3824_CR31",
"unstructured": "Enkhbaatar P, Pruitt BA, Suman O, et al. Pathophysiology, research challenges, and clinical management of smoke inhalation injury. Lancet. 2016;388(10052):1437–46.",
"volume": "388",
"year": "2016"
},
{
"DOI": "10.1016/j.cps.2017.02.009",
"author": "SW Jones",
"doi-asserted-by": "crossref",
"first-page": "505",
"issue": "3",
"journal-title": "Clin Plast Surg",
"key": "3824_CR32",
"unstructured": "Jones SW, Williams FN, Cairns BA, Cartotto R. INHALATION INJURY: pathophysiology, diagnosis, and treatment. Clin Plast Surg. 2017;44(3):505–11.",
"volume": "44",
"year": "2017"
},
{
"DOI": "10.1016/j.jcrc.2013.06.017",
"author": "NM Elsharnouby",
"doi-asserted-by": "crossref",
"first-page": "182.e1-182.e4",
"issue": "1",
"journal-title": "J Crit Care",
"key": "3824_CR33",
"unstructured": "Elsharnouby NM, Eid HEA, Abou Elezz NF, Aboelatta YA. Heparin/N-acetylcysteine: an adjuvant in the management of burn inhalation injury. J Crit Care. 2014;29(1):182.e1-182.e4.",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.1007/s00330-020-07033-y",
"author": "M Francone",
"doi-asserted-by": "crossref",
"first-page": "6808",
"issue": "12",
"journal-title": "Eur Radiol",
"key": "3824_CR34",
"unstructured": "Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808–17.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020200370",
"doi-asserted-by": "crossref",
"key": "3824_CR35",
"unstructured": "Pan F, Ye T, Sun P, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295(3):715–21."
},
{
"DOI": "10.1016/j.ejro.2020.100305",
"author": "Y Yun",
"doi-asserted-by": "crossref",
"first-page": "100305",
"journal-title": "Eur J Radiol Open",
"key": "3824_CR36",
"unstructured": "Yun Y, Wang Y, Hao Y, Xu L, Cai Q. The time course of chest CT lung changes in COVID-19 patients from onset to discharge. Eur J Radiol Open. 2021;8:100305.",
"volume": "8",
"year": "2021"
},
{
"author": "Y Wang",
"first-page": "200843",
"journal-title": "Radiology Published Online March",
"key": "3824_CR37",
"unstructured": "Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study. Radiology Published Online March. 2020;19:200843.",
"volume": "19",
"year": "2020"
},
{
"author": "B Gupta",
"first-page": "222",
"issue": "3",
"journal-title": "Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med",
"key": "3824_CR38",
"unstructured": "Gupta B, Chandrakar S, Gupta N, Jain G. Nebulized heparin to reduce COVID-19-induced acute lung injury: A prospective observational study. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2023;27(3):222–4.",
"volume": "27",
"year": "2023"
},
{
"DOI": "10.1016/j.arres.2021.100015",
"author": "JFSD Lana",
"doi-asserted-by": "crossref",
"first-page": "100015",
"journal-title": "Adv Redox Res",
"key": "3824_CR39",
"unstructured": "Lana JFSD, Lana AVSD, Rodrigues QS, et al. Nebulization of glutathione and N-Acetylcysteine as an adjuvant therapy for COVID-19 onset. Adv Redox Res. 2021;3:100015.",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0294872",
"author": "SY Yu",
"doi-asserted-by": "crossref",
"first-page": "e0294872",
"issue": "11",
"journal-title": "PLoS ONE",
"key": "3824_CR40",
"unstructured": "Yu SY, Choi M, Ryoo S, et al. Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease 2019: A living review and meta-analysis. PLoS ONE. 2023;18(11):e0294872.",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1186/s13052-023-01491-y",
"author": "Y Wang",
"doi-asserted-by": "crossref",
"first-page": "80",
"issue": "1",
"journal-title": "Ital J Pediatr",
"key": "3824_CR41",
"unstructured": "Wang Y, Liu K, Chen C, Zhang C. Acetylcysteine and Budesonide for the treatment of refractory Mycoplasma pneumoniae pneumonia in children: a clinical observation. Ital J Pediatr. 2023;49(1):80.",
"volume": "49",
"year": "2023"
},
{
"DOI": "10.1002/iid3.1068",
"author": "J Chen",
"doi-asserted-by": "crossref",
"first-page": "e1068",
"issue": "11",
"journal-title": "Immun Inflamm Dis",
"key": "3824_CR42",
"unstructured": "Chen J, Zhu Y, Zheng C, Zhao W, Liu Q. Clinical efficacy of Budesonide combined with acetylcysteine in the treatment of mycoplasma pneumonia infection. Immun Inflamm Dis. 2023;11(11):e1068.",
"volume": "11",
"year": "2023"
},
{
"author": "C Wang",
"first-page": "1092",
"issue": "2",
"journal-title": "Int J Clin Exp Med",
"key": "3824_CR43",
"unstructured": "Wang C, Zhang X, Qian W, Liu X, Zhang W. Budesonide combined with Ipratropium bromide in the treatment of Bronchopneumonia and its efficacy on pulmonary function. Int J Clin Exp Med 2020;13(2):1092–7.",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020200490",
"author": "ZY Zu",
"doi-asserted-by": "crossref",
"first-page": "E15",
"issue": "2",
"journal-title": "Radiology",
"key": "3824_CR44",
"unstructured": "Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology. 2020;296(2):E15–25.",
"volume": "296",
"year": "2020"
},
{
"DOI": "10.1093/qjmed/hcad092",
"author": "S O’Reilly",
"doi-asserted-by": "crossref",
"first-page": "750",
"issue": "9",
"journal-title": "QJM",
"key": "3824_CR45",
"unstructured": "O’Reilly S. Pulmonary fibrosis in COVID-19: mechanisms, consequences and targets. QJM. 2023;116(9):750–4.",
"volume": "116",
"year": "2023"
},
{
"DOI": "10.1016/j.pharmthera.2025.108891",
"author": "ECA Vreeman",
"doi-asserted-by": "crossref",
"first-page": "108891",
"journal-title": "Pharmacol Ther",
"key": "3824_CR46",
"unstructured": "Vreeman ECA, Pillay J, Burgess JK. Post-COVID pulmonary sequelae: mechanisms and potential targets to reduce persistent fibrosis. Pharmacol Ther. 2025;272:108891.",
"volume": "272",
"year": "2025"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-025-03824-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of combined nebulization of unfractionated heparin, acetylcysteine, budesonide and ipratropium bromide in hospitalised patients with COVID-19 pneumonia: a randomized controlled clinical trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}
gong2
