Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19
et al., NEJM, May 7, 2020, doi:10.1056/NEJMoa2012410, May 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
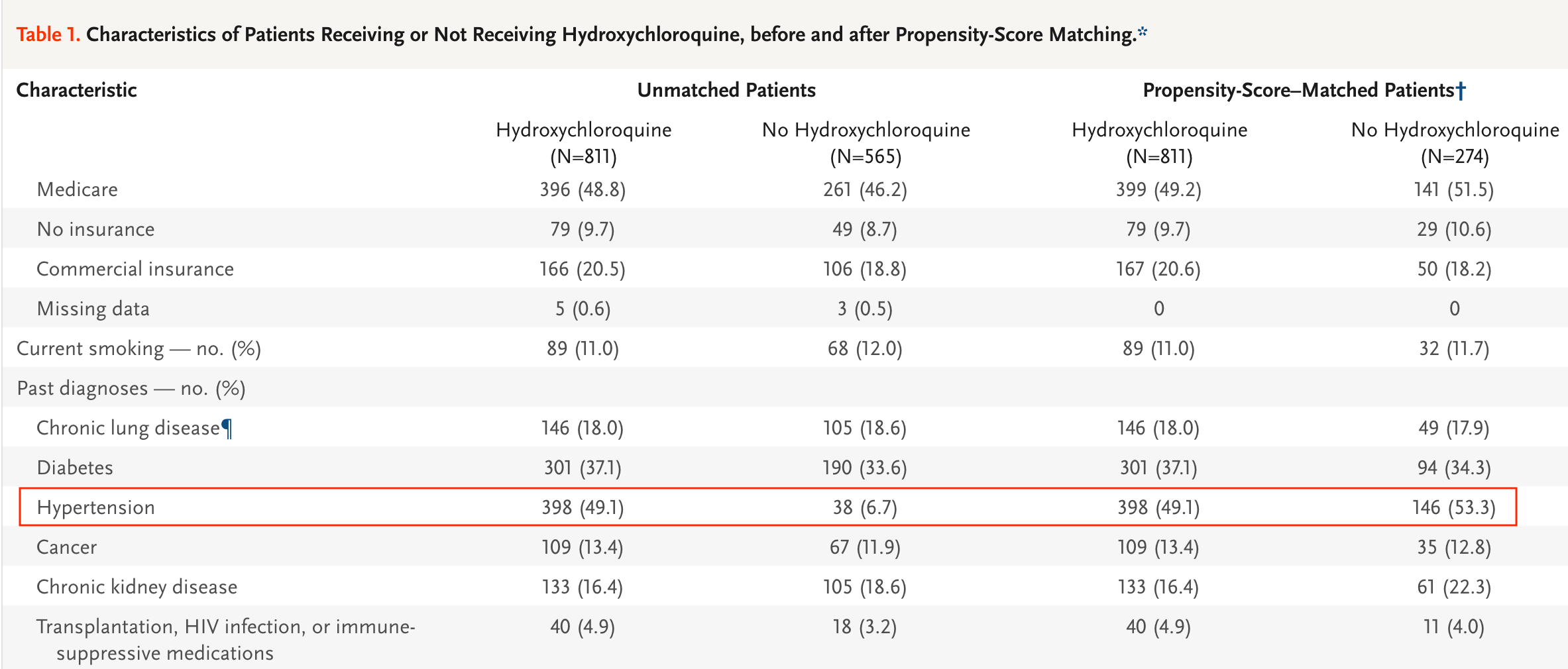
Before propensity matching, 38 control patients had hypertension. After propensity matching, 146 patients had hypertension (Table 1). Even if all propensity matched control patients had hypertension, the control prevalence would only be 14% compared to 49% for treatment. Since patients with hypertension are at much greater risk of mortality (HR 2.12, see1), this appears to invalidate the results.
Observational study of 1,446 hospitalized patients showing no significant effect on a combined intubation/death outcome for late treatment.
However, secondary analysis shows the success of HCQ was hidden by combining intubation and death - death / (combined death/intubation) for HCQ was 60% vs. control 89%, for details see:2.
RCT recommended. No AZ or Zinc. HCQ group much sicker - patients already in mild/moderate ARDS, most of the control group not in ARDS. Control cases received other therapeutics.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments3.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
significant issues found with adjustments.
|
risk of death/intubation, 4.0% higher, HR 1.04, p = 0.76, treatment 262 of 811 (32.3%), control 84 of 565 (14.9%), adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Geleris et al., 7 May 2020, retrospective, USA, peer-reviewed, 12 authors.
Abstract: The
n e w e ng l a n d j o u r na l
of
m e dic i n e
Original Article
Observational Study of Hydroxychloroquine
in Hospitalized Patients with Covid-19
Joshua Geleris, M.D., Yifei Sun, Ph.D., Jonathan Platt, Ph.D., Jason Zucker, M.D.,
Matthew Baldwin, M.D., George Hripcsak, M.D., Angelena Labella, M.D.,
Daniel K. Manson, M.D., Christine Kubin, Pharm.D., R. Graham Barr, M.D., Dr.P.H.,
Magdalena E. Sobieszczyk, M.D., M.P.H., and Neil W. Schluger, M.D.
A BS T R AC T
BACKGROUND
Hydroxychloroquine has been widely administered to patients with Covid-19 without robust evidence supporting its use.
METHODS
We examined the association between hydroxychloroquine use and intubation or
death at a large medical center in New York City. Data were obtained regarding
consecutive patients hospitalized with Covid-19, excluding those who were intubated, died, or discharged within 24 hours after presentation to the emergency
department (study baseline). The primary end point was a composite of intubation
or death in a time-to-event analysis. We compared outcomes in patients who received hydroxychloroquine with those in patients who did not, using a multivariable
Cox model with inverse probability weighting according to the propensity score.
RESULTS
Of 1446 consecutive patients, 70 patients were intubated, died, or discharged within
24 hours after presentation and were excluded from the analysis. Of the remaining
1376 patients, during a median follow-up of 22.5 days, 811 (58.9%) received hydroxychloroquine (600 mg twice on day 1, then 400 mg daily for a median of 5 days);
45.8% of the patients were treated within 24 hours after presentation to the emergency department, and 85.9% within 48 hours. Hydroxychloroquine-treated patients
were more severely ill at baseline than those who did not receive hydroxychloroquine (median ratio of partial pressure of arterial oxygen to the fraction of inspired
oxygen, 223 vs. 360). Overall, 346 patients (25.1%) had a primary end-point event
(180 patients were intubated, of whom 66 subsequently died, and 166 died without
intubation). In the main analysis, there was no significant association between
hydroxychloroquine use and intubation or death (hazard ratio, 1.04, 95% confidence
interval, 0.82 to 1.32). Results were similar in multiple sensitivity analyses.
From the Divisions of General Medicine,
Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L.,
D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the
Departments of Biostatistics (Y.S.) and
Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.),
Vagelos College of Physicians and Surgeons, Columbia University, and New
York–Presbyterian Hospital–Columbia University Irving Medical Center (J.G., J.Z.,
M.B., A.L., D.K.M., C.K.,R.G.B., M.E.S.,
N.W.S.) — all in New York. Address reprint requests to Dr. Schluger at the Division of Pulmonary, Allergy, and Critical
Care Medicine, Columbia University Irving Medical Center, PH-8 E., Rm. 101,
622 W. 168th St., New York, NY 10032, or
at ns311@cumc.columbia.edu.
This article was published on May 7, 2020,
and updated on May 14, 2020, at NEJM.org.
N Engl J Med 2020;382:2411-8.
DOI: 10.1056/NEJMoa2012410
Copyright © 2020 Massachusetts Medical Society.
CONCLUSIONS
In this observational study involving patients with Covid-19 who had been admitted
to the hospital, hydroxychloroquine administration was..
DOI record:
{
"DOI": "10.1056/nejmoa2012410",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2012410",
"alternative-id": [
"10.1056/NEJMoa2012410"
],
"author": [
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Geleris",
"given": "Joshua",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Sun",
"given": "Yifei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Platt",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Zucker",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Baldwin",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Hripcsak",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Labella",
"given": "Angelena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Manson",
"given": "Daniel K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Kubin",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Barr",
"given": "R. Graham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Sobieszczyk",
"given": "Magdalena E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Divisions of General Medicine, Infectious Diseases, and Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine (J.G., J.Z., M.B., A.L., D.K.M., C.K., R.G.B., M.E.S., N.W.S.), the Departments of Biostatistics (Y.S.) and Epidemiology (J.P., R.G.B., N.W.S.), Mailman School of Public Health, and the Department of Biomedical Informatics (G.H.), Vagelos College of Physicians and Surgeons, Columbia University, and New York–Presbyterian Hospital–Columbia University Irving Medical Center ..."
}
],
"family": "Schluger",
"given": "Neil W.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
5,
7
]
],
"date-time": "2020-05-07T21:00:58Z",
"timestamp": 1588885258000
},
"deposited": {
"date-parts": [
[
2020,
6,
17
]
],
"date-time": "2020-06-17T20:57:58Z",
"timestamp": 1592427478000
},
"funder": [
{
"DOI": "10.13039/100000002",
"award": [
"RO1-HL077612",
"RO1-HL093081",
"RO1-HL121270",
"RO1-LM006910"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
},
{
"DOI": "10.13039/100005258",
"award": [
"200-2009-32593"
],
"doi-asserted-by": "publisher",
"name": "National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T07:53:50Z",
"timestamp": 1712562830438
},
"is-referenced-by-count": 1208,
"issue": "25",
"issued": {
"date-parts": [
[
2020,
6,
18
]
]
},
"journal-issue": {
"issue": "25",
"published-print": {
"date-parts": [
[
2020,
6,
18
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
6,
18
]
],
"date-time": "2020-06-18T00:00:00Z",
"timestamp": 1592438400000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2012410",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "2411-2418",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2020,
6,
18
]
]
},
"published-print": {
"date-parts": [
[
2020,
6,
18
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1021/jm0601856",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1016/S0049-0172(10)80012-5",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105938",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105960",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1097/CCM.0000000000002514",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.1016/j.chest.2016.01.003",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.1002/9780470316696",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"author": "Chen Z",
"journal-title": "MedRxiv",
"key": "r13",
"year": "2020"
}
],
"reference-count": 11,
"references-count": 11,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.737908482.793576143",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2012410"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19",
"type": "journal-article",
"volume": "382"
}
