Melatonin in the Prophylaxis of SARS-CoV-2 Infection in Healthcare Workers (MeCOVID): A Randomised Clinical Trial
et al., Journal of Clinical Medicine, doi:10.3390/jcm11041139, MeCOVID, NCT04353128, Feb 2022
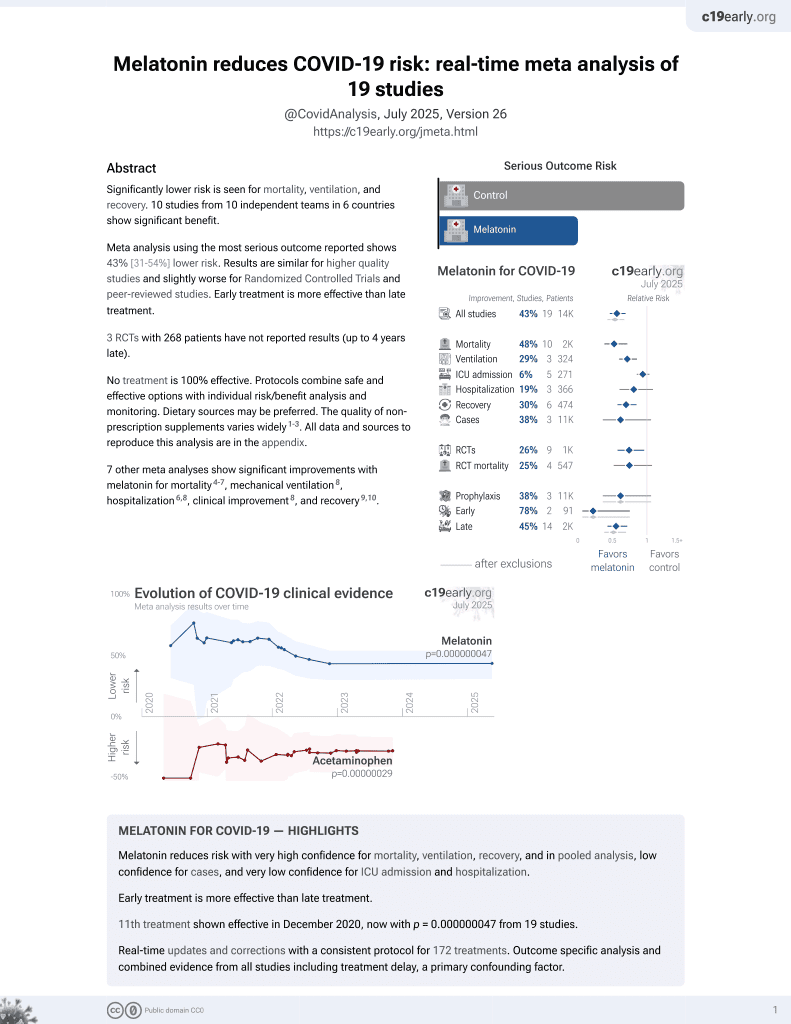
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PrEP RCT healthcare workers in Spain, showing no significant
difference in cases with melatonin prophylaxis. Most cases were asymptomatic
or paucisymtomatic, there were two symptomatic cases, no moderate/severe
cases, and no hospitalization.
The registered primary outcome is symptomatic cases. Authors
report on all cases due to the small number of symptomatic cases. They did not
include the original primary outcome results in the paper, but have provided
the results via email to a contributor.
The dosage in this trial is very low, 2mg daily. Meta
regression suggests higher doses are much more effective. EudraCT
2020-001530-35.
|
risk of symptomatic case, 7.4% lower, RR 0.93, p = 1.00, treatment 1 of 163 (0.6%), control 1 of 151 (0.7%), NNT 2051, primary outcome.
|
|
risk of case, 108.4% higher, RR 2.08, p = 0.26, treatment 9 of 163 (5.5%), control 4 of 151 (2.6%), post-hoc primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
García-García et al., 21 Feb 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, 25 authors, study period April 2020 - December 2020, trial NCT04353128 (history) (MeCOVID).
Melatonin in the Prophylaxis of SARS-CoV-2 Infection in Healthcare Workers (MeCOVID): A Randomised Clinical Trial
We evaluated in this randomised, double-blind clinical trial the efficacy of melatonin as a prophylactic treatment for prevention of SARS-CoV-2 infection among healthcare workers at high risk of SARS-CoV-2 exposure. Healthcare workers fulfilling inclusion criteria were recruited in five hospitals in Spain and were randomised 1:1 to receive melatonin 2 mg administered orally for 12 weeks or placebo. The main outcome was the number of SARS-CoV-2 infections. A total of 344 volunteers were screened, and 314 were randomised: 151 to placebo and 163 to melatonin; 308 received the study treatment (148 placebo; 160 melatonin). We detected 13 SARS-CoV-2 infections, 2.6% in the placebo arm and 5.5% in the melatonin arm (p = 0.200). A total of 294 adverse events were detected in 127 participants (139 in placebo; 155 in melatonin). We found a statistically significant difference in the incidence of adverse events related to treatment: 43 in the placebo arm and 67 in the melatonin arm (p = 0.040), and in the number of participants suffering from somnolence related to treatment: 8.8% (n = 14) in the melatonin versus 1.4% (n = 2) in the placebo arm (p = 0.008). No severe adverse
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest.
References
Abella, Jolkovsky, Biney, Uspal, Hyman et al., Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial, JAMA Intern. Med, doi:10.1001/jamainternmed.2020.6319
Acuña-Castroviejo, Escames, Figueira, De, Oliva et al., Clinical trial to test the efficacy of melatonin in COVID-19, J. Pineal Res, doi:10.1111/jpi.12683
Alavian, Kolahdouzan, Mortezazadeh, Dds, Antiretrovirals for Prophylaxis Against COVID-19: A Comprehensive Literature Review, J. Clin. Pharmacol, doi:10.1002/jcph.1788
Ali, Role of vitamin D in preventing of COVID-19 infection, progression and severity, J. Infect. Public Health, doi:10.1016/j.jiph.2020.06.021
Anderson, Maes, Markus, Rodriguez, Ebola virus: Melatonin as a readily available treatment option, J. Med. Virol, doi:10.1002/jmv.24130
Ayerdi, Puerta, Clavo, Vera, Ballesteros et al., Preventive Efficacy of Tenofovir/Emtricitabine Against Severe Acute Respiratory Syndrome Coronavirus 2 Among Pre-Exposure Prophylaxis Users, Open Forum Infect. Dis, doi:10.1093/ofid/ofaa455
Ben-Zvi, Kivity, Langevitz, Shoenfeld, Hydroxychloroquine: From Malaria to Autoimmunity, Clin. Rev. Allergy Immunol, doi:10.1007/s12016-010-8243-x
Bhattacharya, Patel, Dehari, Agrawal, Singh, Melatonin and its ubiquitous anticancer effects, Mol. Cell. Biochem, doi:10.1007/s11010-019-03617-5
Boga, Coto-Montes, Rosales-Corral, Tan, Reiter, Beneficial actions of melatonin in the management of viral infections: A new use for this "molecular handyman, Rev. Med. Virol, doi:10.1002/rmv.1714
Castilla, Guevara, Miqueleiz, Baigorria, Ibero-Esparza et al., Risk Factors of Infection, Hospitalization and Death from SARS-CoV-2: A Population-Based Cohort Study, J. Clin. Med, doi:10.3390/jcm10122608
Cipolla-Neto, Amaral, Afeche, Tan, Reiter, Melatonin, energy metabolism, and obesity: A review, J. Pineal Res, doi:10.1111/jpi.12137
Claustrat, Leston, Melatonin: Physiological effects in humans, Neurochirurgie, doi:10.1016/j.neuchi.2015.03.002
Clinicaltrials, gov Daily Regimen of Tenofovir/Emtricitabine as Prevention for COVID-19 in Health Care Personnel in Colombia
Clinicaltrials, gov Randomized Clinical Trial for the Prevention of SARS-CoV-2 Infection (COVID-19) in Healthcare Personnel
Clinicaltrials, gov TAF/FTC for Pre-exposure Prophylaxis of COVID-19 in Healthcare Workers (CoviPrep Study
Cruciani, Pati, Masiello, Malena, Pupella et al., Ivermectin for Prophylaxis and Treatment of COVID-19: A Systematic Review and Meta-Analysis, Correction in Diagnostics, doi:10.3390/diagnostics11091645
De Wit, Feldmann, Cronin, Jordan, Okumura et al., Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection, doi:10.1073/pnas.1922083117
Del Amo, Polo, Moreno, Diaz-Brito, Martínez et al., Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy: A Cohort Study, Ann. Intern. Med
Favero, Franceschetti, Bonomini, Rodella, Rezzani, Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation, Int. J. Endocrinol, doi:10.1155/2017/1835195
García, Rubio, Mariblanca, De Soto, García et al., A randomized multicenter clinical trial to evaluate the efficacy of melatonin in the prophylaxis of SARS-CoV-2 infection in high-risk contacts (MeCOVID Trial): A structured summary of a study protocol for a randomised controlled trial, Trials, doi:10.1186/s13063-020-04436-6
Gunn, Middleton, Davies, Revell, Skene, Sex differences in the circadian profiles of melatonin and cortisol in plasma and urine matrices under constant routine conditions, Chronobiol. Int, doi:10.3109/07420528.2015.1112396
Hadizadeh, Supplementation with vitamin D in the COVID-19 pandemic?, Nutr. Rev, doi:10.1093/nutrit/nuaa081
Hellwig, Maia, A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106248
Malin, Suárez, Priesner, Fätkenheuer, Rybniker, Remdesivir against COVID-19 and Other Viral Diseases, Clin. Microbiol. Rev, doi:10.1128/CMR.00162-20
Nainu, Abidin, Bahar, Frediansyah, Bin Emran et al., SARS-CoV-2 reinfection and implications for vaccine development, Hum. Vaccines Immunother, doi:10.1080/21645515.2020.1830683
Preparation, -M.; Writing-Review, Supervision, None
Rajasingham, Bangdiwala, Nicol, Skipper, Pastick et al., Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers: A Randomized Trial, Clin. Infect. Dis, doi:10.1093/cid/ciaa1571
Reiter, Mayo, Tan, Sainz, Alatorre-Jimenez et al., Melatonin as an antioxidant: Under promises but over delivers, J. Pineal Res, doi:10.1111/jpi.12360
Rodríguez-Rubio, Figueira, Acuña-Castroviejo, Borobia, Escames et al., A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): A structured summary of a study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-020-04632-4
Scholtens, Van Munster, Van Kempen, De Rooij, Physiological melatonin levels in healthy older people: A systematic review, J. Psychosom. Res, doi:10.1016/j.jpsychores.2016.05.005
Shiu, Reiter, Tan, Pang, Urgent search for safe and effective treatments of severe acute respiratory syndrome: Is melatonin a promising candidate drug?, J. Pineal Res, doi:10.1034/j.1600-079X.2003.00068.x
Singh, Chauhan, Kakkar, Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2020.173717
Smit, Marinosci, Agoritsas, Calmy, Prophylaxis for COVID-19: A systematic review, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2021.01.013
Sreepadmanabh, Sahu, Chande, COVID-19: Advances in diagnostic tools, treatment strategies, and vaccine development, J. Biosci, doi:10.1007/s12038-020-00114-6
Sun, Gusdon, Qu, Effects of melatonin on cardiovascular diseases: Progress in the past year, Curr. Opin. Lipidol, doi:10.1097/MOL.0000000000000314
Who, Coronavirus Disease
Zhang, Wang, Ni, Di, Ma et al., COVID-19: Melatonin as a potential adjuvant treatment, Life Sci, doi:10.1016/j.lfs.2020.117583
Zhao, Zhao, Ou, Zhang, Lan et al., COVID-19: Coronavirus Vaccine Development Updates, Front. Immunol, doi:10.3389/fimmu.2020.602256
Álvez, SARS-CoV2 coronavirus: So far polite with children. Debatable immunological and non-immunological evidence, Allergol. Immunopathol, doi:10.1016/j.aller.2020.05.003
DOI record:
{
"DOI": "10.3390/jcm11041139",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm11041139",
"abstract": "<jats:p>We evaluated in this randomised, double-blind clinical trial the efficacy of melatonin as a prophylactic treatment for prevention of SARS-CoV-2 infection among healthcare workers at high risk of SARS-CoV-2 exposure. Healthcare workers fulfilling inclusion criteria were recruited in five hospitals in Spain and were randomised 1:1 to receive melatonin 2 mg administered orally for 12 weeks or placebo. The main outcome was the number of SARS-CoV-2 infections. A total of 344 volunteers were screened, and 314 were randomised: 151 to placebo and 163 to melatonin; 308 received the study treatment (148 placebo; 160 melatonin). We detected 13 SARS-CoV-2 infections, 2.6% in the placebo arm and 5.5% in the melatonin arm (p = 0.200). A total of 294 adverse events were detected in 127 participants (139 in placebo; 155 in melatonin). We found a statistically significant difference in the incidence of adverse events related to treatment: 43 in the placebo arm and 67 in the melatonin arm (p = 0.040), and in the number of participants suffering from somnolence related to treatment: 8.8% (n = 14) in the melatonin versus 1.4% (n = 2) in the placebo arm (p = 0.008). No severe adverse events related to treatment were reported. We cannot confirm our hypothesis that administration of melatonin prevents the development of SARS-CoV-2 infection in healthcare workers.</jats:p>",
"alternative-id": [
"jcm11041139"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4831-9374",
"affiliation": [],
"authenticated-orcid": false,
"family": "García-García",
"given": "Irene",
"sequence": "first"
},
{
"affiliation": [],
"family": "Seco-Meseguer",
"given": "Enrique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruiz-Seco",
"given": "Pilar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Navarro-Jimenez",
"given": "Gema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez-Porqueras",
"given": "Raúl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Espinosa-Díaz",
"given": "María",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6207-0712",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ortega-Albás",
"given": "Juan José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sagastagoitia",
"given": "Iñigo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Morales",
"given": "María Teresa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4925-731X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jiménez-González",
"given": "María",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2941-0922",
"affiliation": [],
"authenticated-orcid": false,
"family": "Martínez de Soto",
"given": "Lucía",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bajo-Martínez",
"given": "Ana Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "del Palacio-Tamarit",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López-García",
"given": "Raquel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díaz-García",
"given": "Lucía",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Queiruga-Parada",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giesen",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez-Villena",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Castro-Martínez",
"given": "Marta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3652-002X",
"affiliation": [],
"authenticated-orcid": false,
"family": "González-García",
"given": "Juan J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9145-4718",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rodriguez-Rubio",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de la Oliva",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arribas",
"given": "José R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1823-4174",
"affiliation": [],
"authenticated-orcid": false,
"family": "Carcas",
"given": "Antonio J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8584-3263",
"affiliation": [],
"authenticated-orcid": false,
"family": "Borobia",
"given": "Alberto M.",
"sequence": "additional"
}
],
"container-title": [
"Journal of Clinical Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
22
]
],
"date-time": "2022-02-22T01:47:45Z",
"timestamp": 1645494465000
},
"deposited": {
"date-parts": [
[
2022,
2,
22
]
],
"date-time": "2022-02-22T02:07:30Z",
"timestamp": 1645495650000
},
"indexed": {
"date-parts": [
[
2022,
2,
22
]
],
"date-time": "2022-02-22T02:40:43Z",
"timestamp": 1645497643806
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2077-0383"
}
],
"issue": "4",
"issued": {
"date-parts": [
[
2022,
2,
21
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2022,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
21
]
],
"date-time": "2022-02-21T00:00:00Z",
"timestamp": 1645401600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/11/4/1139/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1139",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
2,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
21
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "Coronavirus Disease 2019 (COVID-19) Situation Report—51www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10"
},
{
"DOI": "10.3389/fimmu.2020.602256",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.3390/jcm10122608",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1080/21645515.2020.1830683",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1007/s12038-020-00114-6",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1007/s11010-019-03617-5",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1016/j.neuchi.2015.03.002",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.lfs.2020.117583",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1111/jpi.12360",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1155/2017/1835195",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1034/j.1600-079X.2003.00068.x",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1002/jmv.24130",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1002/rmv.1714",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1111/jpi.12683",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1186/s13063-020-04632-4",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.jpsychores.2016.05.005",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.3109/07420528.2015.1112396",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1111/jpi.12137",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1097/MOL.0000000000000314",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.aller.2020.05.003",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1186/s13063-020-04436-6",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"key": "ref22",
"unstructured": "Coronavirus Disease 2019 (COVID-19)https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-january-2022"
},
{
"DOI": "10.1016/j.ejphar.2020.173717",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1007/s12016-010-8243-x",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1001/jamainternmed.2020.6319",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1093/cid/ciaa1571",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/j.cmi.2021.01.013",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1002/jcph.1788",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.7326/M20-3689",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1093/ofid/ofaa455",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"key": "ref31"
},
{
"key": "ref32"
},
{
"key": "ref33"
},
{
"DOI": "10.1073/pnas.1922083117",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1128/CMR.00162-20",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106248",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3390/diagnostics11091645",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1093/nutrit/nuaa081",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1016/j.jiph.2020.06.021",
"doi-asserted-by": "publisher",
"key": "ref39"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"score": 1,
"short-container-title": [
"JCM"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Melatonin in the Prophylaxis of SARS-CoV-2 Infection in Healthcare Workers (MeCOVID): A Randomised Clinical Trial"
],
"type": "journal-article",
"volume": "11"
}