Assessment of vitamin D deficiency and COVID-19 diagnosis in patients with breast or prostate cancer using electronic medical records
et al., Journal of Clinical Oncology, doi:10.1200/JCO.2021.39.15_suppl.6589, May 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
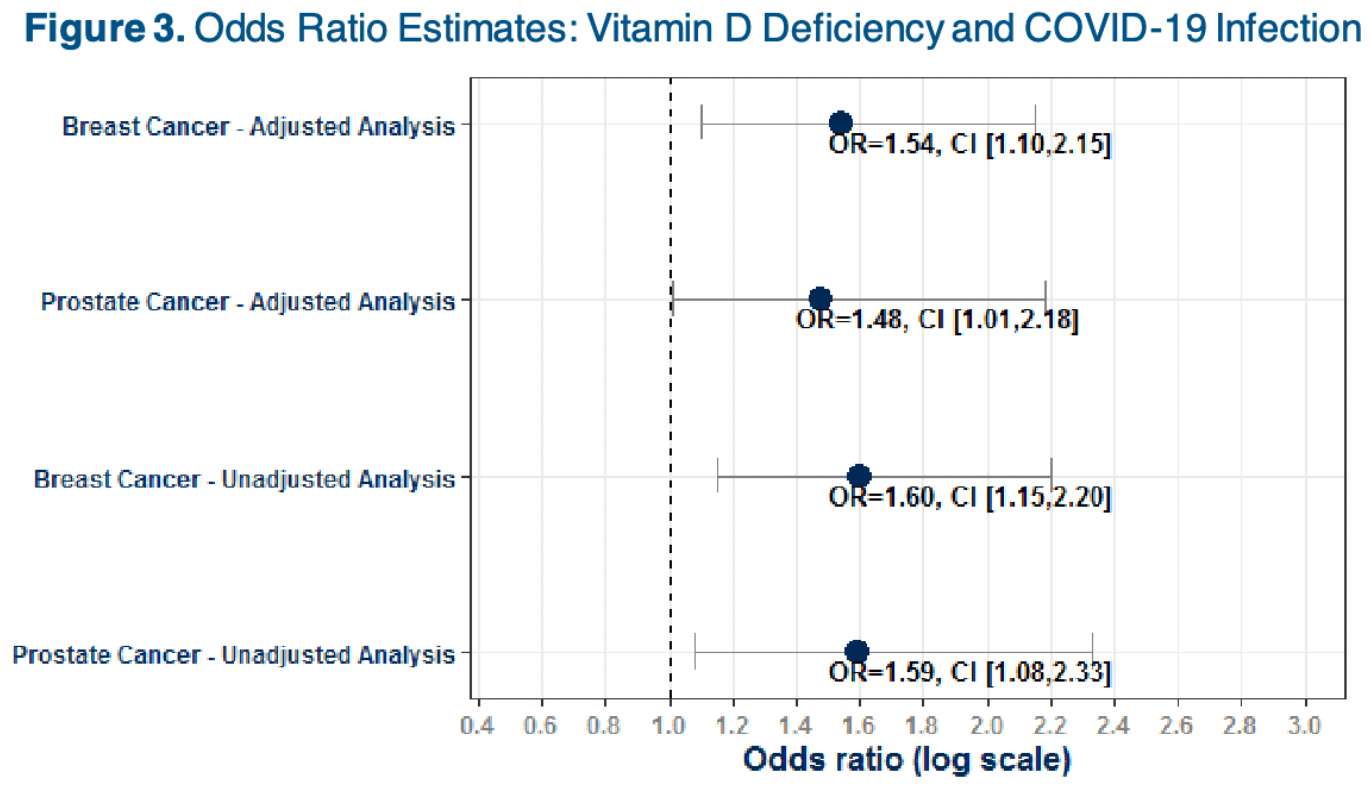
Retrospective 16,287 breast cancer and 14,919 prostate cancer showing increased risk of COVID-19 cases with vitamin D deficiency.
This is the 68th of 228 COVID-19 sufficiency studies for vitamin D, which collectively show higher levels reduce risk with p<0.0000000001.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of case, 35.1% lower, OR 0.65, p = 0.01, high D levels 13,903, low D levels 2,384, adjusted per study, inverted to make OR<1 favor high D levels, breast cancer patients, logistic regression, RR approximated with OR.
|
|
risk of case, 32.4% lower, OR 0.68, p = 0.045, high D levels 13,601, low D levels 1,318, adjusted per study, inverted to make OR<1 favor high D levels, prostate cancer patients, logistic regression, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Galaznik et al., 28 May 2021, retrospective, USA, preprint, 6 authors.
Abstract: Assessment of Vitamin D deficiency and COVID-19 diagnosis in patients with breast or
prostate cancer using Electronic Medical Records
Aaron Galaznik, MD MBA1 | Emelly Rusli, MPH1 | Vicki Wing, MS1 | Rahul Jain, PhD1 | Sheila Diamond, MS CGC1 | David Fajgenbaum, MD MBA MSc FCPP2
1 Medidata Acorn AI, a Dassault Systèmes company, New York, NY, 10014 | 2 Castleman Disease Collaborative Network and the University of Pennsylvania, Philadelphia, PA, 19104
RESULTS
BACKGROUND
BACKGROUND
•
•
While patients with cancer are known to be at increased risk
of infection in part due to the immunocompromising nature of
cancer treatments, recent data indicate a particularly high
risk for COVID-19 infection and poor outcomes. 1
•
Our study suggests
potentially vulnerable
populations, such as breast
and prostate cancer
patients, may have an
elevated risk of
COVID-19 infection if
vitamin D deficient.
Vitamin D deficiency has been previously reported in two
leading causes of cancer deaths: breast and prostate. 4
•
In this study, we performed a retrospective cohort analysis on
nationally representative electronic medical records (EMR) to
assess whether vitamin D deficiency affects risk of COVID19 among these patients.
Vitamin D may play an important role in COVID-19. A recent
study demonstrated vitamin D deficiency may increase risk of
COVID-19 infection, and a small randomized controlled trial
in Spain reported significant improvement in mortality among
hospitalized patients treated with calcifediol. 2,3
METHODS
Figure 1. Study Timeline
•
A total of 16,287 breast cancer and 14,919 prostate cancer patients were included in the study. (Figure 2)
Table 1. Patient Demographic and Clinical Characteristics
Breast Cancer
TOTAL (N = 16,287)
Patient Characteristics
N/Mean
%/SD
N/Mean
%/SD
68.9
11.3
73.6
8.5
<70 years (n, %)
7,962
48.9%
4,625
31.0%
70-79 years (n, %)
5,368
33.0%
6,499
43.6%
80+ years (n, %)
2,957
18.2%
3,795
25.4%
16,287
100.0%
0
0.0%
0
13,805
2,102
305
16
49
10
0.0%
84.8%
12.9%
1.9%
0.1%
0.3%
0.1%
14,919
12,390
2,405
89
12
22
1
100.0%
83.1%
16.1%
0.6%
0.1%
0.1%
0.0%
2,384
14.6%
1,318
8.8%
1.1
1.5
1.4
1.7
Congestive heart failure (n, %)
1,075
6.60%
1,483
9.94%
Obesity (n, %)
5,036
30.9%
4,627
31.0%
Diabetes mellitus (n, %)
3,327
20.4%
3,897
26.1%
356
2.2%
303
2.0%
1,730
10.6%
2,371
15.9%
Age (Mean, SD)
Sex (n, %)
Female
Male
White
Race (n, %)
Black
Asian
Native Hawaiian/Pacific Islander
American Indian or Alaska Native
Missing
Vitamin D deficient (n, %)
Comorbid
Conditions
Quan Charlson Comorbidity Index (Mean, SD)
Liver disease (n, %)
Figure 2. Patient Attrition
Patient with ≥ 1 encounter
between 3/1/2018 and
3/1/2019, and after 3/1/2020
(index date)
Age ≥ 18 and non-missing sex
and race
n = 1,630,384 (52.8%)
•
•
•
Patients with breast (female) or prostate (male) cancer
were identified between 3/1/2018 and 3/1/2020 from
Healthjump EMR data provided pro-bono by the COVID-19
Research Database.5
Logistic regressions, adjusted for baseline demographic
and clinical characteristics assessed in the 12 months prior
to 3/1/2020, were conducted to estimate the effect of
•
•
The average age was 68.9 years in the breast cancer cohort
and 73.6 years in the prostate cancer cohort.
(Table 1)
•
Approximately 15% of the breast cancer cohort and 9% of
the prostate cancer cohort had vitamin D deficiency.
•
The most common comorbid conditions were obesity
(approximately a third..
DOI record:
{
"DOI": "10.1200/jco.2021.39.15_suppl.6589",
"ISSN": [
"0732-183X",
"1527-7755"
],
"URL": "http://dx.doi.org/10.1200/jco.2021.39.15_suppl.6589",
"abstract": "<jats:p> 6589 </jats:p><jats:p> Background: While patients with cancer are known to be at increased risk of infection in part due to the immunocompromising nature of cancer treatments, recent data indicate a particularly high risk for COVID-19 infection and poor outcomes (Wang et al., 2020). A recent study (Meltzer et al., 2020) demonstrated Vitamin D deficiency may increase risk of COVID-19 infection, and a small randomized controlled trial in Spain reported significant improvement in mortality among hospitalized patients treated with calcifediol. Vitamin D deficiency has been reported in two leading causes of cancer deaths: breast and prostate. In this study, we performed a retrospective cohort analysis on nationally representative electronic medical records (EMR) to assess whether Vitamin D deficiency affects risk of COVID-19 among these patients. Methods: Patients with breast (female) or prostate (male) cancer were identified between 3/1/2018 and 3/1/2020 from EMR data provided pro-bono by the COVID-19 Research Database ( covid19researchdatabase.org ). Patients with an ICD-10 code for Vitamin D deficiency or < 20ng/mL 20(OH)D laboratory result within 12 months prior to 3/1/2020 were classified as Vitamin D deficient. COVID-19 diagnosis was defined using ICD-10 codes and laboratory results for COVID-19 at any time after 3/1/2020. Logistic regressions, adjusting for baseline demographic and clinical characteristics, were conducted to estimate the effect of Vitamin D deficiency on COVID-19 incidence in each cancer cohort. Results: A total of 16,287 breast cancer and 14,919 prostate cancer patients were included in the study. The average age was 68.9 years in the breast cancer cohort and 73.6 years in the prostate cancer cohort. The breast cancer cohort consisted of 85% Whites, 13% Black or African Americans, and less than 5% of other races. A similar race distribution was observed in the prostate cancer cohort. Unadjusted analysis showed the risk of COVID-19 was higher among Vitamin D deficient patients compared to non-deficient patients in both cohorts (breast: OR = 1.60 [95% C.I.: 1.15, 2.20]; prostate: OR = 1.59 [95% C.I.: 1.08, 2.33]). Similar findings were observed when assessed in subgroups of patients with newly diagnosed cancer in the dataset, as well as after adjusting for baseline characteristics. Conclusions: Our study suggests breast and prostate cancer patients may have an elevated risk of COVID-19 infection if Vitamin D deficient. These results support findings by Meltzer et al., 2020 demonstrating a relationship between Vitamin D deficiency and COVID-19 infection. While a randomized clinical trial is warranted to confirm the role for Vitamin D supplementation in preventing COVID-19, our study underscores the importance of monitoring Vitamin D levels across and within cancer populations, particularly in the midst of the global COVID-19 pandemic. </jats:p>",
"alternative-id": [
"10.1200/JCO.2021.39.15_suppl.6589"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2021-05-28"
}
],
"author": [
{
"affiliation": [
{
"name": "Acorn AI By Medidata, a Dassault Systèmes Company, New York, NY;"
}
],
"family": "Galaznik",
"given": "Aaron",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Acorn AI By Medidata, a Dassault Systèmes Company, New York, NY;"
}
],
"family": "Rusli",
"given": "Emelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Acorn AI By Medidata, a Dassault Systèmes Company, New York, NY;"
}
],
"family": "Wing",
"given": "Vicki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Acorn AI By Medidata, a Dassault Systèmes Company, New York, NY;"
}
],
"family": "Jain",
"given": "Rahul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Acorn AI By Medidata, a Dassault Systèmes Company, New York, NY;"
}
],
"family": "Diamond",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Castleman Disease Collaborative Network and the University of Pennsylvania, Philadelphia, PA;"
}
],
"family": "Fajgenbaum",
"given": "David",
"sequence": "additional"
}
],
"container-title": [
"Journal of Clinical Oncology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ascopubs.org"
]
},
"created": {
"date-parts": [
[
2021,
6,
2
]
],
"date-time": "2021-06-02T14:28:38Z",
"timestamp": 1622644118000
},
"deposited": {
"date-parts": [
[
2021,
6,
3
]
],
"date-time": "2021-06-03T17:38:14Z",
"timestamp": 1622741894000
},
"funder": [
{
"name": "None"
}
],
"indexed": {
"date-parts": [
[
2021,
12,
16
]
],
"date-time": "2021-12-16T01:13:47Z",
"timestamp": 1639617227727
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0732-183X"
},
{
"type": "electronic",
"value": "1527-7755"
}
],
"issue": "15_suppl",
"issued": {
"date-parts": [
[
2021,
5,
20
]
]
},
"journal-issue": {
"issue": "15_suppl",
"published-print": {
"date-parts": [
[
2021,
5,
20
]
]
}
},
"language": "en",
"member": "233",
"original-title": [],
"page": "6589-6589",
"prefix": "10.1200",
"published": {
"date-parts": [
[
2021,
5,
20
]
]
},
"published-print": {
"date-parts": [
[
2021,
5,
20
]
]
},
"publisher": "American Society of Clinical Oncology (ASCO)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"JCO"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Cancer Research",
"Oncology"
],
"subtitle": [],
"title": [
"Assessment of vitamin D deficiency and COVID-19 diagnosis in patients with breast or prostate cancer using electronic medical records."
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1200/crossmark",
"volume": "39"
}
