Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.101242, NCT04359095, Jul 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
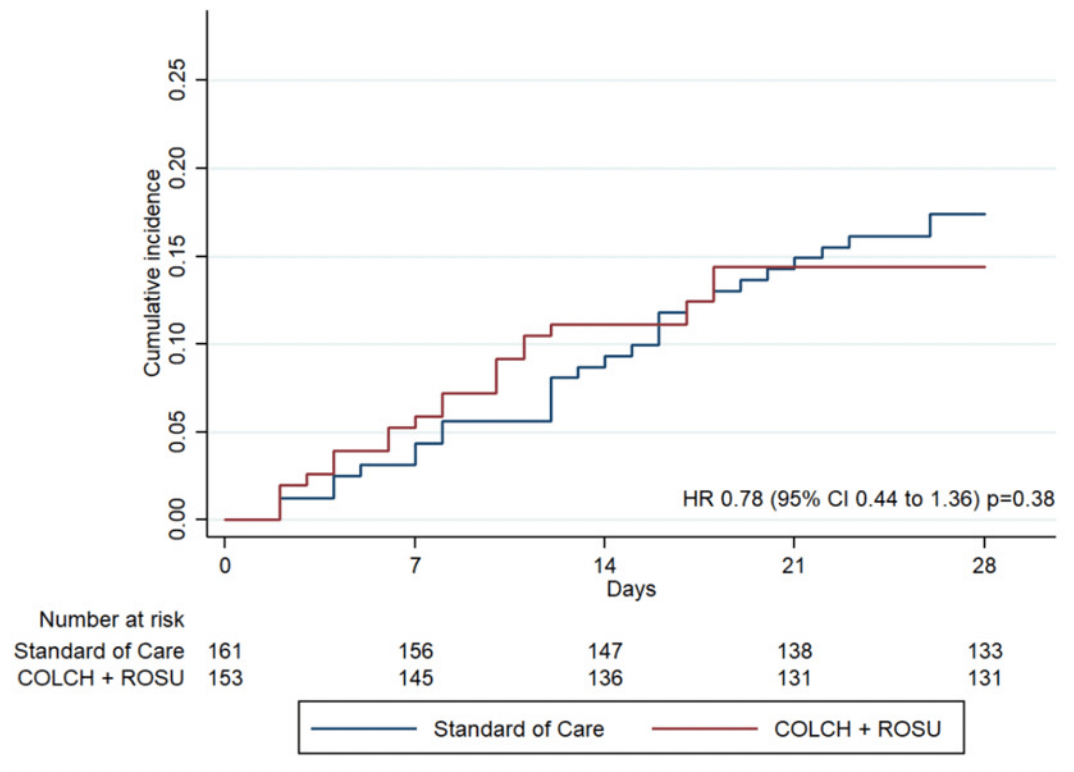
RCT 633 hospitalized patients in Colombia, 153 treated with colchicine + rosuvastatin, 160 treated with emtricitabine + tenofovir, and 159 treated with the combination of both regimens. Statistically significant improvement was seen only for the combination of emtricitabine + tenofovir + rosuvastatin + colchicine.
Although the 22% lower mortality is not statistically significant, it is consistent with the significant 22% lower mortality [12‑31%] from meta-analysis of the 41 mortality results to date.
|
risk of death, 22.0% lower, HR 0.78, p = 0.38, treatment 22 of 153 (14.4%), control 28 of 161 (17.4%), NNT 33, adjusted per study, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gaitán-Duarte et al., 10 Jul 2021, Randomized Controlled Trial, Colombia, peer-reviewed, 17 authors, study period 24 August, 2020 - 20 March, 2021, average treatment delay 10.0 days, dosage 0.5mg days 1-14, this trial uses multiple treatments in the treatment arm (combined with rosuvastatin) - results of individual treatments may vary, trial NCT04359095 (history).
Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial
eClinicalMedicine, doi:10.1016/j.eclinm.2021.101242
Background The use of rosuvastatin plus colchicine and emtricitabine/tenofovir in hospitalized patients with SARS-CoV-2 disease (COVID-19) has not been assessed. The objective of this study was to assess the effectiveness and safety of rosuvastatin plus colchicine, emtricitabine/tenofovir, and their combined use in these patients. Methods This was a randomized, controlled, open-label, multicentre, parallel, pragmatic study conducted in six referral hospitals in Bogot a, Colombia. The study enrolled hospitalized patients over 18 years of age with a confirmed diagnosis of COVID-19 complicated with pneumonia, not on chronic treatment with the study medications, and with no contraindications for their use. Patients were assigned 1:1:1:1. 1) emtricitabine with tenofovir disoproxil fumarate (FTC/TDF, 200/300 mg given orally for 10 days); 2) colchicine plus rosuvastatin (COLCH+ROSU, 0.5 mg and 40 mg given orally for 14 days); 3) emtricitabine with tenofovir disoproxil plus colchicine and rosuvastatin at the same doses and for the same period of time (FTC/TDF+COLCH+ROSU); or 4) the Colombian consensus standard of care, including a corticosteroid (SOC). The primary endpoint was 28-day all-cause mortality. A modified intention-to-treat analysis was used together with a usefulness analysis to determine which could be the best treatment. The trial was registered at ClinicalTrials.gov: NCT04359095
Author's contributions Hernando G. Gait an-Duarte: leader researcher, design, contact with external entities, data collection, analysis and interpretation, funding, writing; Carlos Alvarez-Moreno: design, data collection, analysis and interpretation; Carlos Javier Rincon: design, analysis and interpretation, writing; Nancy Yomayusa-Gonz alez: data collection, analysis and interpretation, writing; Jorge Alberto Cort es: data collection, analysis and interpretation, writing; Juan Carlos Villar: design, data collection; Juan Sebasti an Bravo-Ojeda: data collection; Angel Garc ıa-Peña: design, data collection; Wilson Adarme-Jaimes: design, data collection; Viviana Rodr ıguez-Romero: design, analysis and interpretation; Steffany L Villate-Soto: supervisory role; Giancarlo Buitrago: design, data analysis and interpretation, writing; Julio Chac on-Sarmiento: data collection; Mart ın Mac ıas-Quintero: data analysis; Claudia Patricia Vaca: analysis and interpretation; Carlos G omez-Restrepo: analysis and interpretation; Nelcy Rodr ıguez-Malag on: design, data collection, analysis and interpretation.
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j. eclinm.2021.101242.
References
Austin, A Tutorial on Multilevel Survival Analysis: Methods, Models and Applications, Int Stat Rev
Bastard, Rosen, Zhang, Autoantibodies against type I IFNs in patients with life-threatening COVID-19, Science
Borba, Val, Sampaio, Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial, JAMA Netw Open
Chien, Anderson, Jockusch, Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19, J Proteome Res
Clososki, Soldi, Da Silva, Tenofovir Disoproxil Fumarate: New Chemical Developments and Encouraging in vitro Biological Results for SARS-CoV-2, J Braz Chem Soc
Deftereos, Giannopoulos, Vrachatis, Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Netw Open
Del Amo, Polo, Moreno, Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy : A Cohort Study, Ann Intern Med
Gravel, Opatrny, Shapiro, The intention-to-treat approach in randomized controlled trials: are authors saying what they do and doing what they say?, Clin Trials
Grifoni, Weiskopf, Ramirez, Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals, Cell
Group, Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Higgins, Thomas, Chandler, Cochrane handbook for systematic reviews of interventions
Horby, Campbell, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Infectious Diseases, doi:10.1101/2021.05.18.21257267
Inspiration Investigatorssadeghipour, Talasaz, Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial, JAMA
Melchjorsen, Risør, Søgaard, Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells, J Acquir Immune Defic Syndr
Minz, Bansal, Kasliwal, Statins and SARS-CoV-2 disease: Current concepts and possible benefits, Diabetes Metab Syndr
N Eant, Lingas, Hingrat, Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort, Proc Natl Acad Sci U S A
Parienti, Prazuck, Peyro-Saint-Paul, Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial, EClinicalMedicine
Perico, Ostermann, Bontempeill, Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation, J Am Soc Nephrol
Permana, Huang, Purwiga, In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis, Pharmacol Rep
R€ Ucker, Schwarzer, Ranking treatments in frequentist network meta-analysis works without resampling methods, BMC Med Res Methodol
Saavedra-Trujillo, Consenso Colombiano de atenci on, diagn ostico y manejo de la infecci on por SARS-COV-2/COVID-19 en establecimientos de atenci on de la salud: recomendaciones basadas en consenso de expertos e informadas en la evidencia ACIN-IETS
Schwartz, Lellouch, Explanatory and pragmatic attitudes in therapeutical trials, J Clin Epidemiol
Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ
Takemoto, Liao, Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors, Arterioscler Thromb Vasc Biol
Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med
Vahedian-Azimi, Mohammadi, Beni, Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis, Arch Med Sci
Varga, Flammer, Steiger, Endothelial cell infection and endotheliitis in COVID-19, Lancet
Vickerstaff, Omar, Ambler, Methods to adjust for multiple comparisons in the analysis and sample size calculation of randomised controlled trials with multiple primary outcomes, BMC Med Res Methodol
Vitiello, Ferrara, Colchicine and SARS-CoV-2: Management of the hyperinflammatory state, Respir Med
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Who, Pan, Peto, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Wilson, Runciman, Gibberd, Harrison, Newby et al., The Quality in Australian Health Care Study, Med J Aust
Xing, Tu, Liu, Efficacy and safety of COVID-19 vaccines: a systematic review, Zhongguo Dang Dai Er Ke Za Zhi
Zhang, Bastard, Liu, Inborn errors of type I IFN immunity in patients with life-threatening COVID-19, Science
DOI record:
{
"DOI": "10.1016/j.eclinm.2021.101242",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2021.101242",
"alternative-id": [
"S258953702100523X"
],
"article-number": "101242",
"author": [
{
"affiliation": [],
"family": "Gaitán-Duarte",
"given": "H.G.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Álvarez-Moreno",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rincón-Rodríguez",
"given": "C.J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yomayusa-González",
"given": "N.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0882-9652",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cortés",
"given": "J.A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villar",
"given": "J.C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bravo-Ojeda",
"given": "J.S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Peña",
"given": "A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7401-223X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Adarme-Jaimes",
"given": "W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez-Romero",
"given": "V.A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villate-Soto",
"given": "S.L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buitrago",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chacón-Sarmiento",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Macias-Quintero",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaca",
"given": "C.P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Restrepo",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez-Malagón",
"given": "N.",
"sequence": "additional"
}
],
"container-title": [
"eClinicalMedicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
20
]
],
"date-time": "2021-12-20T17:57:55Z",
"timestamp": 1640023075000
},
"deposited": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T22:34:04Z",
"timestamp": 1643927644000
},
"indexed": {
"date-parts": [
[
2022,
2,
4
]
],
"date-time": "2022-02-04T23:40:22Z",
"timestamp": 1644018022751
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2589-5370"
}
],
"issued": {
"date-parts": [
[
2022,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
2
]
],
"date-time": "2021-12-02T00:00:00Z",
"timestamp": 1638403200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S258953702100523X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S258953702100523X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101242",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.eclinm.2021.101242_bib0001",
"series-title": "Global Economic Prospects, June 2021",
"year": "2021"
},
{
"article-title": "Efficacy and safety of COVID-19 vaccines: a systematic review",
"author": "Xing",
"first-page": "221",
"journal-title": "Zhongguo Dang Dai Er Ke Za Zhi",
"key": "10.1016/j.eclinm.2021.101242_bib0002",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m2980",
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"doi-asserted-by": "crossref",
"first-page": "m2980",
"journal-title": "BMJ",
"key": "10.1016/j.eclinm.2021.101242_bib0003",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results",
"author": "Solidarity Trial Consortium",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101242_bib0004",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101242_bib0005",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.rmed.2021.106322",
"article-title": "Colchicine and SARS-CoV-2: Management of the hyperinflammatory state",
"author": "Vitiello",
"doi-asserted-by": "crossref",
"journal-title": "Respir Med",
"key": "10.1016/j.eclinm.2021.101242_bib0006",
"volume": "178",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2020.10.021",
"article-title": "Statins and SARS-CoV-2 disease: Current concepts and possible benefits",
"author": "Minz",
"doi-asserted-by": "crossref",
"first-page": "2063",
"journal-title": "Diabetes Metab Syndr",
"key": "10.1016/j.eclinm.2021.101242_bib0007",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1021/acs.jproteome.0c00392",
"article-title": "Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19",
"author": "Chien",
"doi-asserted-by": "crossref",
"first-page": "4690",
"journal-title": "J Proteome Res",
"key": "10.1016/j.eclinm.2021.101242_bib0008",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial",
"author": "Deftereos",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.eclinm.2021.101242_bib0009",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1007/s43440-021-00233-3",
"article-title": "In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis",
"author": "Permana",
"doi-asserted-by": "crossref",
"first-page": "769",
"journal-title": "Pharmacol Rep",
"key": "10.1016/j.eclinm.2021.101242_bib0010",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.7326/M20-3689",
"article-title": "Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy : A Cohort Study",
"author": "Del Amo",
"doi-asserted-by": "crossref",
"first-page": "536",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.eclinm.2021.101242_bib0011",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1016/j.jclinepi.2009.01.012",
"article-title": "Explanatory and pragmatic attitudes in therapeutical trials",
"author": "Schwartz",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/j.eclinm.2021.101242_bib0012",
"volume": "62",
"year": "2009"
},
{
"article-title": "Consenso Colombiano de atención, diagnóstico y manejo de la infección por SARS-COV-2/COVID-19 en establecimientos de atención de la salud: recomendaciones basadas en consenso de expertos e informadas en la evidencia ACIN-IETS. Segunda Edición",
"author": "Saavedra-Trujillo",
"first-page": "262",
"journal-title": "Infectio",
"key": "10.1016/j.eclinm.2021.101242_bib0013",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.5694/j.1326-5377.1995.tb124691.x",
"article-title": "The Quality in Australian Health Care Study",
"author": "Wilson",
"doi-asserted-by": "crossref",
"first-page": "458",
"journal-title": "Med J Aust",
"key": "10.1016/j.eclinm.2021.101242_bib0014",
"volume": "163",
"year": "1995"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2021.101242_bib0015",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"article-title": "Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial",
"author": "Borba",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.eclinm.2021.101242_bib0016",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1186/s12874-019-0754-4",
"article-title": "Methods to adjust for multiple comparisons in the analysis and sample size calculation of randomised controlled trials with multiple primary outcomes",
"author": "Vickerstaff",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "BMC Med Res Methodol",
"key": "10.1016/j.eclinm.2021.101242_bib0017",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1177/1740774507081223",
"article-title": "The intention-to-treat approach in randomized controlled trials: are authors saying what they do and doing what they say?",
"author": "Gravel",
"doi-asserted-by": "crossref",
"first-page": "350",
"journal-title": "Clin Trials",
"key": "10.1016/j.eclinm.2021.101242_bib0018",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.1111/insr.12214",
"article-title": "A Tutorial on Multilevel Survival Analysis: Methods, Models and Applications",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Int Stat Rev",
"key": "10.1016/j.eclinm.2021.101242_bib0019",
"volume": "85",
"year": "2017"
},
{
"DOI": "10.1002/9781119536604",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.101242_bib0020",
"unstructured": "Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. 2020 10.1002/9781119536604 (accessed Aug 18, 2021)."
},
{
"DOI": "10.1186/s12874-015-0060-8",
"article-title": "Ranking treatments in frequentist network meta-analysis works without resampling methods",
"author": "Rücker",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "BMC Med Res Methodol",
"key": "10.1016/j.eclinm.2021.101242_bib0021",
"volume": "15",
"year": "2015"
},
{
"DOI": "10.1016/j.eclinm.2021.100993",
"article-title": "Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial",
"author": "Parienti",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.eclinm.2021.101242_bib0022",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.4152",
"author": "Sadeghipour",
"doi-asserted-by": "crossref",
"first-page": "1620",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.101242_bib0023",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.5114/aoms/132950",
"article-title": "Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis",
"author": "Vahedian-Azimi",
"doi-asserted-by": "crossref",
"first-page": "579",
"journal-title": "Arch Med Sci",
"key": "10.1016/j.eclinm.2021.101242_bib0024",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.eclinm.2021.101242_bib0025",
"year": "2021"
},
{
"DOI": "10.1101/2021.05.18.21257267",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.101242_bib0026",
"unstructured": "RECOVERY Collaborative Group, Horby PW, Campbell M, et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Infectious Diseases (except HIV/AIDS), 2021 DOI: 10.1101/2021.05.18.21257267."
},
{
"DOI": "10.1126/science.abd4570",
"article-title": "Inborn errors of type I IFN immunity in patients with life-threatening COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "eabd4570",
"journal-title": "Science",
"key": "10.1016/j.eclinm.2021.101242_bib0027",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1126/science.abd4585",
"article-title": "Autoantibodies against type I IFNs in patients with life-threatening COVID-19",
"author": "Bastard",
"doi-asserted-by": "crossref",
"first-page": "eabd4585",
"journal-title": "Science",
"key": "10.1016/j.eclinm.2021.101242_bib0028",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.05.015",
"article-title": "Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals",
"author": "Grifoni",
"doi-asserted-by": "crossref",
"first-page": "1489",
"journal-title": "Cell",
"key": "10.1016/j.eclinm.2021.101242_bib0029",
"volume": "181",
"year": "2020"
},
{
"article-title": "Tenofovir Disoproxil Fumarate: New Chemical Developments and Encouraging in vitro Biological Results for SARS-CoV-2",
"author": "Clososki",
"first-page": "1552",
"journal-title": "J Braz Chem Soc",
"key": "10.1016/j.eclinm.2021.101242_bib0030",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2017962118",
"article-title": "Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort",
"author": "Néant",
"doi-asserted-by": "crossref",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.eclinm.2021.101242_bib0031",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1097/QAI.0b013e3182185276",
"article-title": "Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells",
"author": "Melchjorsen",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "J Acquir Immune Defic Syndr",
"key": "10.1016/j.eclinm.2021.101242_bib0032",
"volume": "57",
"year": "2011"
},
{
"DOI": "10.1681/ASN.V74594",
"article-title": "Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation",
"author": "Perico",
"doi-asserted-by": "crossref",
"first-page": "594",
"journal-title": "J Am Soc Nephrol",
"key": "10.1016/j.eclinm.2021.101242_bib0033",
"volume": "7",
"year": "1996"
},
{
"DOI": "10.1161/hq1101.098486",
"article-title": "Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors",
"author": "Takemoto",
"doi-asserted-by": "crossref",
"first-page": "1712",
"journal-title": "Arterioscler Thromb Vasc Biol",
"key": "10.1016/j.eclinm.2021.101242_bib0034",
"volume": "21",
"year": "2001"
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"article-title": "Endothelial cell infection and endotheliitis in COVID-19",
"author": "Varga",
"doi-asserted-by": "crossref",
"first-page": "1417",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2021.101242_bib0035",
"volume": "395",
"year": "2020"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"score": 1,
"short-container-title": [
"eClinicalMedicine"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial"
],
"type": "journal-article",
"volume": "43"
}
