Efficacy and safety of nitazoxanide combined with ritonavir-boosted atazanavir for the treatment of mild to moderate COVID-19
et al., medRxiv, doi:10.1101/2022.02.03.22270152, NCT04459286, Feb 2022
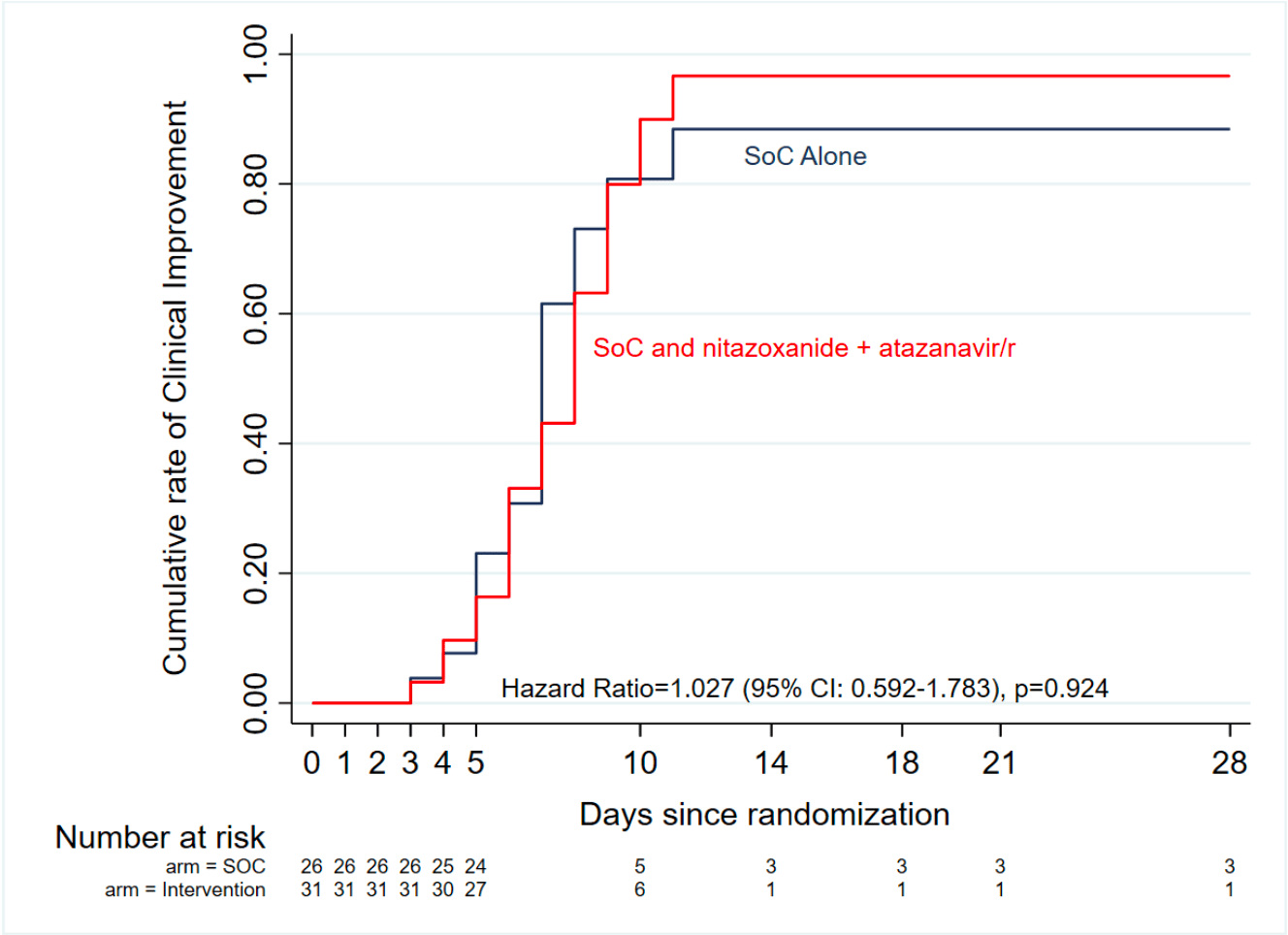
Small RCT in Nigeria with 31 nitazoxanide and atazanavir/ritonavir patients, and 26 control patients, showing no significant differences with treatment. 4 treatment group patients discontinued treatment due to the size of the tablets. Time from onset is not provided, only time from diagnosis. NACOVID. 14-day course of nitazoxanide (1000 mg b.i.d.) and atazanavir/ritonavir (300/100 mg od). NCT04459286 (history).
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
|
risk of no recovery, 11.4% higher, HR 1.11, p = 0.72, treatment 31, control 26, inverted to make HR<1 favor treatment, time to clinical improvement, Cox proportional hazards, primary outcome.
|
|
risk of no recovery, 86.9% higher, HR 1.87, p = 0.10, treatment 31, control 26, inverted to make HR<1 favor treatment, time to symptom resolution, Cox proportional hazards.
|
|
viral load, 5.2% lower, relative load 0.95, p = 0.92, treatment 31, control 26, viral load change from days 2 to 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Fowotade et al., 4 Feb 2022, Randomized Controlled Trial, Nigeria, preprint, 18 authors, study period 25 November, 2020 - 20 April, 2021, this trial uses multiple treatments in the treatment arm (combined with atazanavir/ritonavir) - results of individual treatments may vary, trial NCT04459286 (history).
Efficacy and safety of nitazoxanide combined with ritonavir-boosted atazanavir for the treatment of mild to moderate COVID-19
doi:10.1101/2022.02.03.22270152
Background Finding effective therapeutics for COVID-19 continues to be an urgent need, especially considering use context limitations and high cost of currently approved agents. The NACOVID trial investigated the efficacy and safety of repurposed antiprotozoal and antiretroviral drugs, nitazoxanide and atazanavir/ritonavir, used in combination for COVID-19.
Methods In this pilot, randomized, open-label trial conducted in Nigeria, patients diagnosed with mild to moderate COVID-19 were randomly assigned to receive standard of care (SoC) or SoC plus a 14-day course of nitazoxanide (1000 mg b.i.d.) and atazanavir/ritonavir (300/100 mg od) and followed through day 28. Study endpoints included time to clinical improvement, SARS-CoV-2 viral load change, and time to complete symptom resolution. Safety and pharmacokinetics of nitazoxanide active metabolite, tizoxanide, were also evaluated. This trial was registered with ClinicalTrials.gov
(NCT04459286). Findings There was no difference in time to clinical improvement between the SoC (n = 26) and SoC plus intervention arms (n = 31; Cox proportional hazards regression analysis adjusted hazard ratio, aHR = 0.898, 95% CI: 0.492-1.638, p = 0.725). No difference was observed in the pattern of saliva SARS-CoV-2 viral load changes from days 2 to 28 in the 35% of patients with detectable virus at baseline (20/57) between the two arms (aHR = 0.948, 95% CI: 0.341-2.636, p = 0.919). There was no significant difference in time from enrolment to complete symptom resolution (aHR = 0.535, 95% CI: 0.251 -1.140, p = 0.105). Atazanavir/ritonavir increased tizoxanide plasma exposure by 68% and median trough plasma concentration was 1546 ng/ml (95% CI: 797-2557), above its putative EC90 in 54% of patients. Tizoxanide was not detectable in saliva. Interpretation These findings should be interpreted in the context of incomplete enrolment (64%) and the limited number of patients with detectable SARS-CoV-2 in saliva at baseline in this trial. Funding The University of Liverpool. indicated poor penetration into the respiratory tracts. Specifically, there were no differences in time to clinical improvement, viral load changes, and symptom resolutions between patients who were given standard of care alone and those who combined it with study intervention.
Implications of all the available evidence The clinical benefit of nitazoxanide remains uncertain. The present study highlights the need for early insight into target site biodistribution of potential COVID-19 therapeutics to better inform candidate selection for clinical trials.
Declaration of interests We declare no competing interests.
Contributors AdO, AnO and SR conceived the initial study. AdO designed the study and developed the protocol with input from all authors. AF, FB, BE, and BOA were responsible for study enrolment and data acquisition. CH was responsible for SARS-CoV-2 viral load determination using RT-PCR. AdO, AA, BA and OOB were responsible for database management and pharmacokinetic analyses. AdO, AA, AF and BE verified the underlying data. AFF and AdO were responsible for analysis and interpretation of data. AdO drafted the manuscript. AnO and OOB critically revised the manuscript. All authors contributed to conducting the trial. All authors revised the report and read and approved the final version before submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
Arshad, Pertinez, Box, Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics, Clin Pharmacol Ther
Boffito, Back, Flexner, Consensus on Correct Interpretation of Protein Binding in Plasma and Other Biological Matrices for COVID-19 Therapeutic Development, Clin Pharmacol Ther
Callahan, Ditelberg, Dutta, Saliva is Comparable to Nasopharyngeal Swabs for Molecular Detection of SARS-CoV-2, Microbiol Spectr
Cao, Forrest, Zhang, A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs, Antiviral Res
Cucinotta, Vanelli, WHO Declares COVID-19 a Pandemic, Acta Biomed
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis
Eedara, Alabsi, Encinas-Basurto, Polt, Ledford et al., Inhalation Delivery for the Treatment and Prevention of COVID-19 Infection, Pharmaceutics
Elalfy, Besheer, El-Mesery, Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19, J Med Virol
Fehr, Perlman, Coronaviruses: An Overview of Their Replication and Pathogenesis, Coronaviruses
Fintelman-Rodrigues, Sacramento, Lima, Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production, bioRxiv
Griffiths, Fitzgerald, Jaki, ACCORD: A Randomized, Multicentre, Seamless, Adaptive Phase I/II Platform Study to Determine the Optimal Dose, Safety and Efficacy of Multiple Candidate Agents for the Treatment of COVID-19: A structured summary of a study protocol for a randomised platform trial, Trials
Haffizulla, Hartman, Hoppers, Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis
Hanson, Barker, Hillyard, Self-Collected Anterior Nasal and Saliva Specimens versus Health Care Worker-Collected Nasopharyngeal Swabs for the Molecular Detection of SARS-CoV-2, J Clin Microbiol
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Hiscox, Khoo, Stewart, Owen, Shutting the gate before the horse has bolted: is it time for a conversation about SARS-CoV-2 and antiviral drug resistance?, J Antimicrob Chemother
Li, Liu, Shi, Wang, Xue et al., Tissue-Specific Proteomics Analysis of Anti-COVID-19
Lu, Stratton, Tang, Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle, J Med Virol
Lu, Zhao, Li, Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, The Lancet
Marshall, Murthy, Diaz, A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis
Olagunju, Fowotade, Olagunoye, Efficacy and safety of nitazoxanide plus atazanavir/ritonavir for the treatment of moderate to severe COVID-19 (NACOVID): A structured summary of a study protocol for a randomised controlled trial, Trials
Patil, Saraswat, Patki, Kunda, Patel, Aerosolized nanoliposomal carrier of remdesivir: an effective alternative for COVID-19 treatment in vitro, Nanomedicine (Lond)
Rajoli, Pertinez, Arshad, Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, Br J Clin Pharmacol
Rocco, Silva, Cruz, Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial, Eur Respir J
Rossignol, Matthew, Oaks, Early treatment with nitazoxanide prevents worsening of mild and moderate COVID-19 and subsequent hospitalization, medRxiv
Silva, Espejo, Pereyra, Efficacy of Nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, pilot study, medRxiv
Thorlund, Dron, Park, Hsu, Forrest et al., A real-time dashboard of clinical trials for COVID-19, Lancet Digit Health
Tilmanis, Van Baalen, Oh, Rossignol, Hurt, The susceptibility of circulating human influenza viruses to tizoxanide, the active metabolite of nitazoxanide, Antiviral Res
Uddin, Shirin, Hossain, Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting, Microbiol Spectr
Van Den Elsen, Oostenbrink, Heysell, Systematic Review of Salivary Versus Blood Concentrations of Antituberculosis Drugs and Their Potential for Salivary Therapeutic Drug Monitoring, Ther Drug Monit
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Xu, Zhao, Teng, Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV, Viruses
Yee, Truong, Pannaraj, Saliva Is a Promising Alternative Specimen for the Detection of SARS-CoV-2 in Children and Adults, J Clin Microbiol
Zhang, Chando, Everett, Patten, Dehal et al., In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of property to in vivo bilirubin glucuronidation, Drug Metab Dispos: The Biological Fate of Chemicals
DOI record:
{
"DOI": "10.1101/2022.02.03.22270152",
"URL": "http://dx.doi.org/10.1101/2022.02.03.22270152",
"abstract": "<jats:p>Background: Finding effective therapeutics for COVID-19 continues to be an urgent need, especially considering use context limitations and cost of currently approved agents. The NACOVID trial investigated the efficacy and safety of repurposed antiprotozoal and antiretroviral drugs, nitazoxanide and atazanavir/ritonavir, used in combination for COVID-19.\n\nMethods: In this pilot, randomized, open label trial conducted in Nigeria, patients diagnosed with mild to moderate COVID-19 were randomly assigned to receive standard of care (SoC) or SoC plus 14-day course of nitazoxanide (1000 mg b.i.d.) and atazanavir/ritonavir (300/100 mg od) and followed through day 28. Study endpoints included time to clinical improvement, SARS-CoV-2 viral load change, and time to complete symptoms resolution. Safety and pharmacokinetics of nitazoxanide active metabolite, tizoxanide, were also evaluated. This trial was registered with ClinicalTrials.gov (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04459286\">NCT04459286</jats:ext-link>).\n\nFindings: There was no difference in time to clinical improvement between the SoC (n = 26) and SoC plus intervention arms (n = 31; Cox proportional hazards regression analysis adjusted hazard ratio, aHR = 0.898, 95% CI: 0.492-1.638, p = 0.725). No difference was observed in the pattern of saliva SARS-CoV-2 viral load changes from days 2 to 28 in the 35% of patients with detectable virus at baseline (20/57) between the two arms (aHR = 0.948, 95% CI: 0.341-2.636, p = 0.919). There was no significant difference in time from enrolment to complete symptoms resolution (aHR = 0.535, 95% CI: 0.251 -1.140, p = 0.105). Atazanavir/ritonavir increased tizoxanide plasma exposure by 68% and median trough plasma concentration was 1546 ng/ml (95% CI: 797-2557), above its putative EC90 in 54% of patients. Tizoxanide was not detectable in saliva.\n\nInterpretation: These findings should be interpreted in the context of incomplete enrolment (64%) and the limited number of patients with detectable SARS-CoV-2 in saliva at baseline in this trial.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
2,
4
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0224-7600",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fowotade",
"given": "Adeola",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bamidele",
"given": "Folasade",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Egbetola",
"given": "Boluwatife",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adefuye",
"given": "Bolanle Olufunlola",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9184-8258",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fagbamigbe",
"given": "Adeniyi Francis",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6722-1970",
"affiliation": [],
"authenticated-orcid": false,
"family": "Adeagbo",
"given": "Babatunde Ayodeji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olagunoye",
"given": "Ajibola",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1899-5213",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ojo",
"given": "Temitope Olumuyiwa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adebiyi",
"given": "Akindele Olupelumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olagunju",
"given": "Omobolanle Ibitayo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ladipo",
"given": "Olabode Taiwo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akinloye",
"given": "Abdulafeez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onayade",
"given": "Adedeji",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8295-7150",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bolaji",
"given": "Oluseye Oladotun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3056-6705",
"affiliation": [],
"authenticated-orcid": false,
"family": "Happi",
"given": "Christian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6946-1097",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rannard",
"given": "Steve",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9819-7651",
"affiliation": [],
"authenticated-orcid": false,
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6588-5749",
"affiliation": [],
"authenticated-orcid": false,
"family": "Olagunju",
"given": "Adeniyi",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
5
]
],
"date-time": "2022-02-05T06:30:17Z",
"timestamp": 1644042617000
},
"deposited": {
"date-parts": [
[
2022,
2,
5
]
],
"date-time": "2022-02-05T06:30:17Z",
"timestamp": 1644042617000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
2,
5
]
],
"date-time": "2022-02-05T07:11:24Z",
"timestamp": 1644045084347
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
2,
4
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.02.03.22270152",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
2,
4
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
2,
4
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Efficacy and safety of nitazoxanide combined with ritonavir-boosted atazanavir for the treatment of mild to moderate COVID-19"
],
"type": "posted-content"
}