Sep 2 2025 |
et al., medRxiv, doi:10.1101/2025.08.29.25334732 | Long-term follow-up of treatment comparisons in RECOVERY: a randomised, open-label, platform trial for patients hospitalised with COVID-19 |
| 6-month followup of RECOVERY patients. Results are reported within the respective trials for each treatment. | ||
Dec 31 2023 |
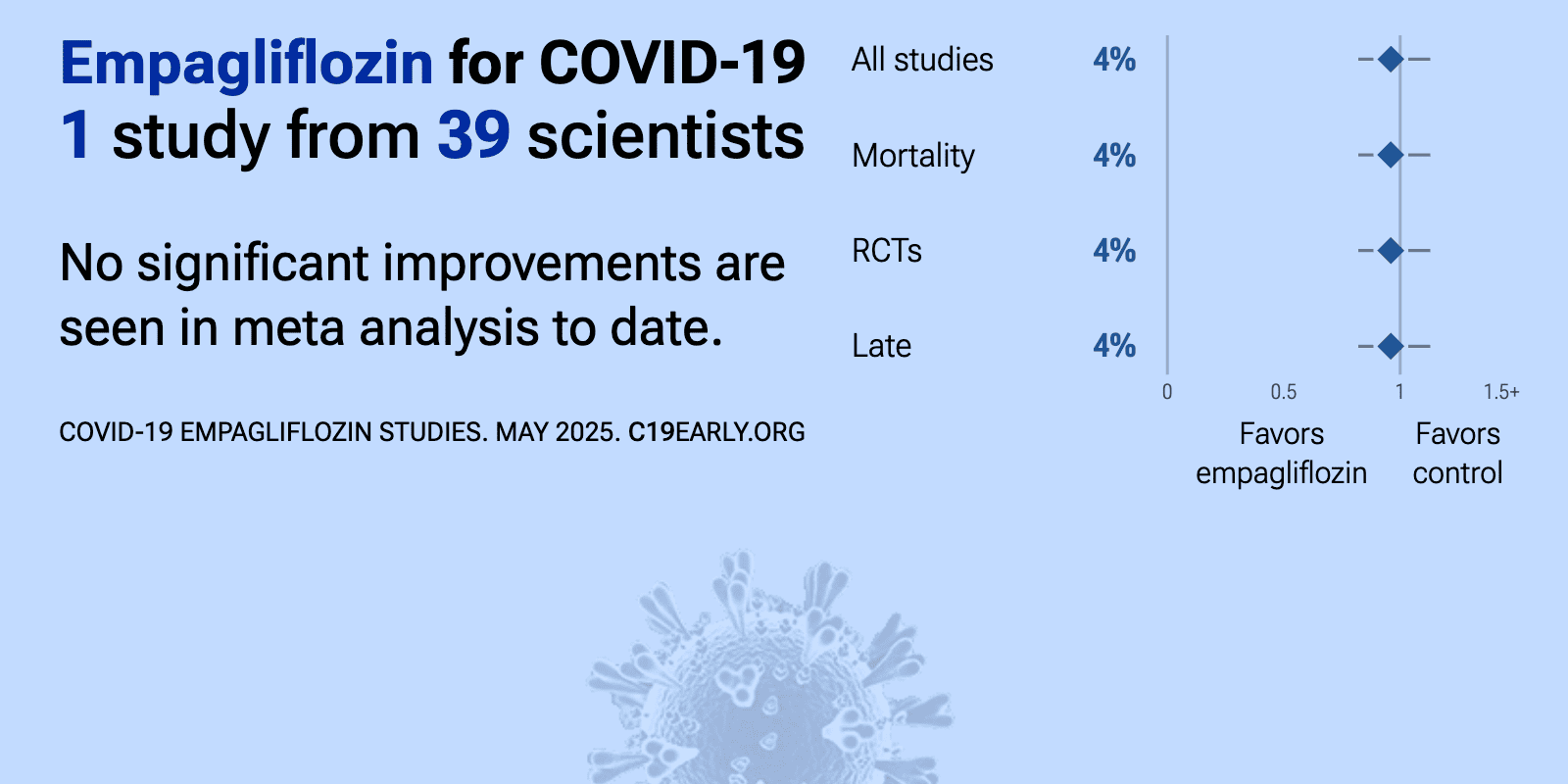
et al., The Lancet Diabetes & Endocrinology, doi:10.1016/S2213-8587(23)00253-X | Empagliflozin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial |
| 4% lower mortality (p=0.59), 3% lower ventilation (p=0.77), and 3% higher hospital discharge (p=0.44). RCT 4,271 hospitalized COVID-19 patients showing no significant difference in outcomes with empagliflozin treatment. 6-month results are from [Horby] | ||