Effect of Preadmission Proton Pump Inhibitor (PPI) on the clinical outcome of Covid-19 Hospitalised Patients during the Pandemic
et al., Academic Journal of Gastroenterology & Hepatology, doi:10.33552/AJGH.2023.03.000568, Nov 2023
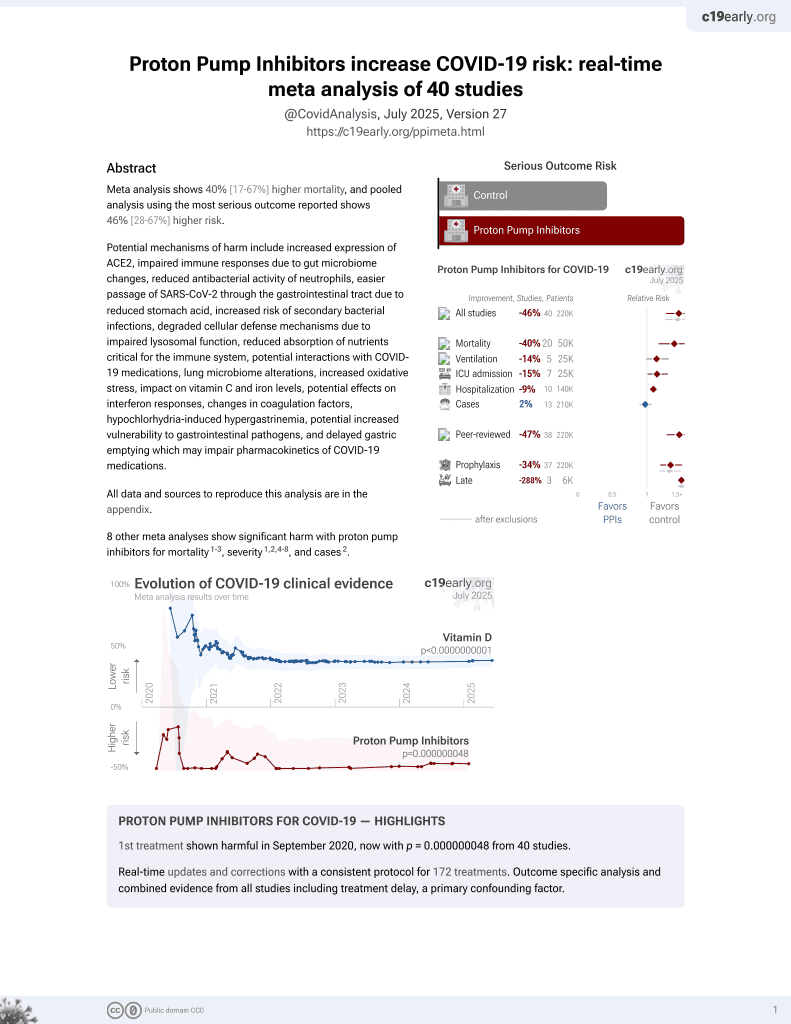
PPIs for COVID-19
1st treatment shown to increase risk in
September 2020, now with p = 0.000000048 from 40 studies.
6,400+ studies for
210+ treatments. c19early.org
|
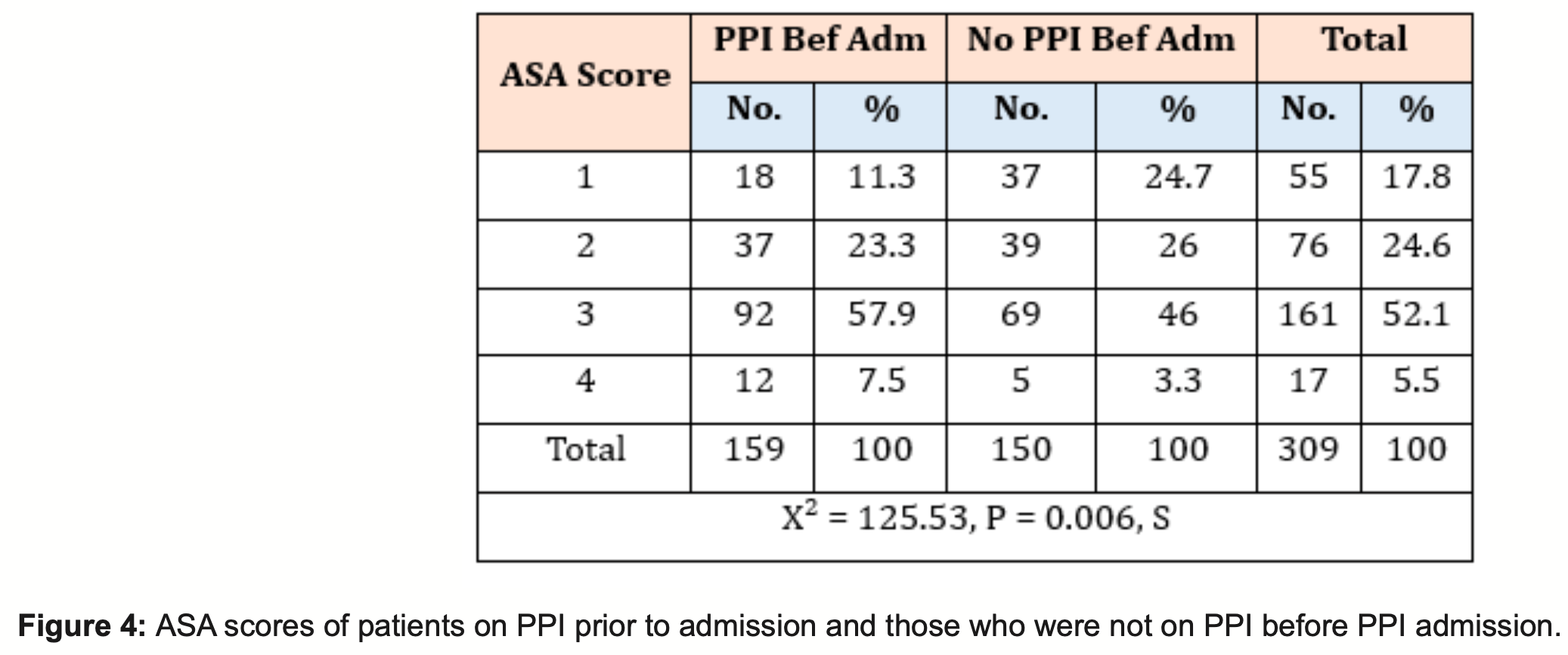
Retrospective 309 hospitalized patients showing higher risk of severe cases (ASA≥3) with PPI use.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 17.1% higher, RR 1.17, p = 0.49, treatment 36 of 159 (22.6%), control 29 of 150 (19.3%).
|
|
risk of ICU admission, 24.9% higher, RR 1.25, p = 0.05, treatment 90 of 159 (56.6%), control 68 of 150 (45.3%).
|
|
ASA 4, 126.4% higher, RR 2.26, p = 0.14, treatment 12 of 159 (7.5%), control 5 of 150 (3.3%).
|
|
ASA ≥3, 32.6% higher, RR 1.33, p = 0.006, treatment 104 of 159 (65.4%), control 74 of 150 (49.3%).
|
|
hospitalization time, 19.0% lower, relative time 0.81, p = 0.18, treatment mean 9.8 (±13.1) n=159, control mean 12.1 (±16.6) n=150.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Elkanzi et al., 17 Nov 2023, retrospective, United Kingdom, peer-reviewed, 8 authors.
Effect of Preadmission Proton Pump Inhibitor (PPI) on the clinical outcome of Covid-19 Hospitalised Patients during the Pandemic
doi:10.33552/AJGH.2023.03.000568
COVID-19 has proved to be a severe disease since the end of 2019; according to WHO, over 600 million people have contracted this infection [1] . Most people who contracted this disease showed no symptoms. Initially, the mean hospitalisation period for symptomatic patients was 16 days, ranging between 3 to 45 days [2] . A significant complication of COVID-19 was found to be respiratory hypoxaemia, which accounted for up to 75% of hospital admissions secondary to pneumonia, along with acute respiratory disease (ARDS). While prodromal systems, such as fever, cough and shortness of breath, were seen in most symptomatic Covid patients, many system involvements were also observed. For instance, 19.7% of patients were found to have a cardiac injury [3] . Similarly, many systems involvements have been observed in SARS patients: 36.4% showed neurological manifestations of their disease [4], 19% had Abstract Methodology: Prospectively captured observational data was analyzed to include patients (>18 yr.) at the hospital with COVID-19 infection. PPI data was derived from hospital and primary care records, and the study period is between February 2020 and February 2021. Clinical outcomes of COVID-19 patients who were on proton pump inhibitors preadmission were compared with those of COVID-19 patients who were not on proton pump inhibitors simultaneously. The study's primary endpoint was 60-day mortality, intensive care unit admission, high dependency unit admission, and the development of COVID-19 complications. Additional endpoints included the length of critical care admission.
Study type: Observational Cohort Study. Results: 309 patients were evaluated in this study; 159 were on proton pump inhibitors, and 150 were not on proton pump inhibitors at index admission. The mean length of stay was 9.8 in the PPI group and 12.1 in the non-PPI group. A slightly increased mortality rate of 22.6% in the PPI group compared with 19.3 % in the non-PPI group. Intensive Care Unit (ITU) and High Dependency Unit (HDU) admissions were higher in the PPI group (56.6%,30.8% respectively) than in the non-PPI group (45.3%,20.7%). Complications were more common in the PPI group74.8% had pulmonary complications, and 3.1% had thromboembolic complications. In the non-PPI group, 54% had pulmonary complications, which was over 20 % less than in the PPI group, 6% had thromboembolic complications, 1.93 times more than the PPI group.
Conclusion: In Our study, PPI usage at index admission succeeded in showing worsening of outcomes in Covid 19 hospitalised patients, similar to recently published papers. This proposed causation needs further evaluation via well-conducted prospective studies.
are observational and thus, causality cannot be established. This proposed causation needs further evaluation via well-conducted prospective studies.
References
Aguila, Cua, Repurposed GI drugs in the treatment of COVID-19, Dig Dis Sci
Almario, Chey, Spiegel, Increased risk of COVID-19 among users of proton pump inhibitors, Am J Gastroenterol
Bojkova, Mcgreig, Mclaughlin, SARS-CoV-2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles, BioRxiv, doi:10.1101/2020.04.03.024257v1
Charpiat, Bleyzac, Tod, Proton pump inhibitors are risk factors for viral infections: even for COVID-19?, Clin Drug Investig
Darnell, Subbarao, Feinstone, Taylor, Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV, J Virol Methods
Fatima, Almas, Lakhani, Jahangir, Ahmed, The use of proton pump inhibitors and COVID-19: A systematic review and meta-analysis, Tropical Medicine and Infectious Disease
Fisher, Fisher, Acid-suppressive therapy and risk of infections: pros and cons, Clin Drug Investig
Fossmark, Martinsen, Waldum, Adverse effects of proton pump inhibitors-evidence and plausibility, International Journal of Molecular Sciences
Hayase, Tobita, Sato, Detection of type B influenza virus genes from biopsied gastric mucosa, J Gastroenterol
Homolak, Kodvanj, Widely available lysosome targeting agents should be considered as potential therapy for COVID-19, Int J Antimicrob Agents
Lee, Ha, Yeniova, Moon, Kim, Severe clinical outcomes of COVID-19 associated with Proton Pump Inhibitors: A nationwide cohort study with propensity score matching, Gut
Luxenburger, Sturm, Biever, Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor?, J Intern Med
Mao, Qiu, He, Tan, Li, Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis, The Lancet Gastroenterology & Hepatology
Mao, Wang, Hu, Chen, Neurologic manifestations of hospitalised patients with coronavirus disease 2019 in Wuhan, China, JAMA Neurology
Piazza, Campia, Hurwitz, Snyder, Rizzo, Registry of arterial and venous thromboembolic complications in patients with COVID-19, Journal of the American College of Cardiology
Prag, Prag, Fredlund, Proton pump inhibitors as a risk factor for norovirus infection, Epidemiol Infect
Ramachandran, Perisetti, Gajendran, Prehospitalisation proton pump inhibitor (PPI) use and clinical outcomes in COVID-19, Eur J Gastroenterol Hepatol
Shi, Qin, Shen, Cai, Liu, Association of cardiac injury with mortality in hospitalised patients with COVID-19 in Wuhan, China, JAMA Cardiology
Tastemur, Ataseven, Is it possible to use proton pump inhibitors in COVID-19 treatment and prophylaxis?, Med Hypotheses
Vesper, Jawdi, Altman, Haines, Tao, The effect of proton pump inhibitors on the human microbiota, Current Drug Metabolism
Vilcu, Sabatte, Blanchon, Association between acute gastroenteritis and continuous use of proton pump inhibitors during winter periods of highest circulation of enteric viruses, JAMA Netw Open
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (covid-19), JAMA
Zhang, Wu, Ling, Analysis of the effect of proton pump inhibitors on the course of common COVID-19, J Inflamm Res
Zhou, Li, Zhao, Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus, Sci Adv