Virion-Independent Extracellular Vesicle (EV)-Dependent Transmission of SARS-CoV-2 as a Potential New Mechanism of Viral RNA Spread in Human Cells
et al., Viruses, doi:10.3390/v18010145, Jan 2026
In vitro study showing that extracellular vesicles (EVs) can transmit SARS-CoV-2 replicon RNA between cells independently of infectious virus particles.
Ekmen et al., 22 Jan 2026, peer-reviewed, 7 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Virion-Independent Extracellular Vesicle (EV)-Dependent Transmission of SARS-CoV-2 as a Potential New Mechanism of Viral RNA Spread in Human Cells
Viruses, doi:10.3390/v18010145
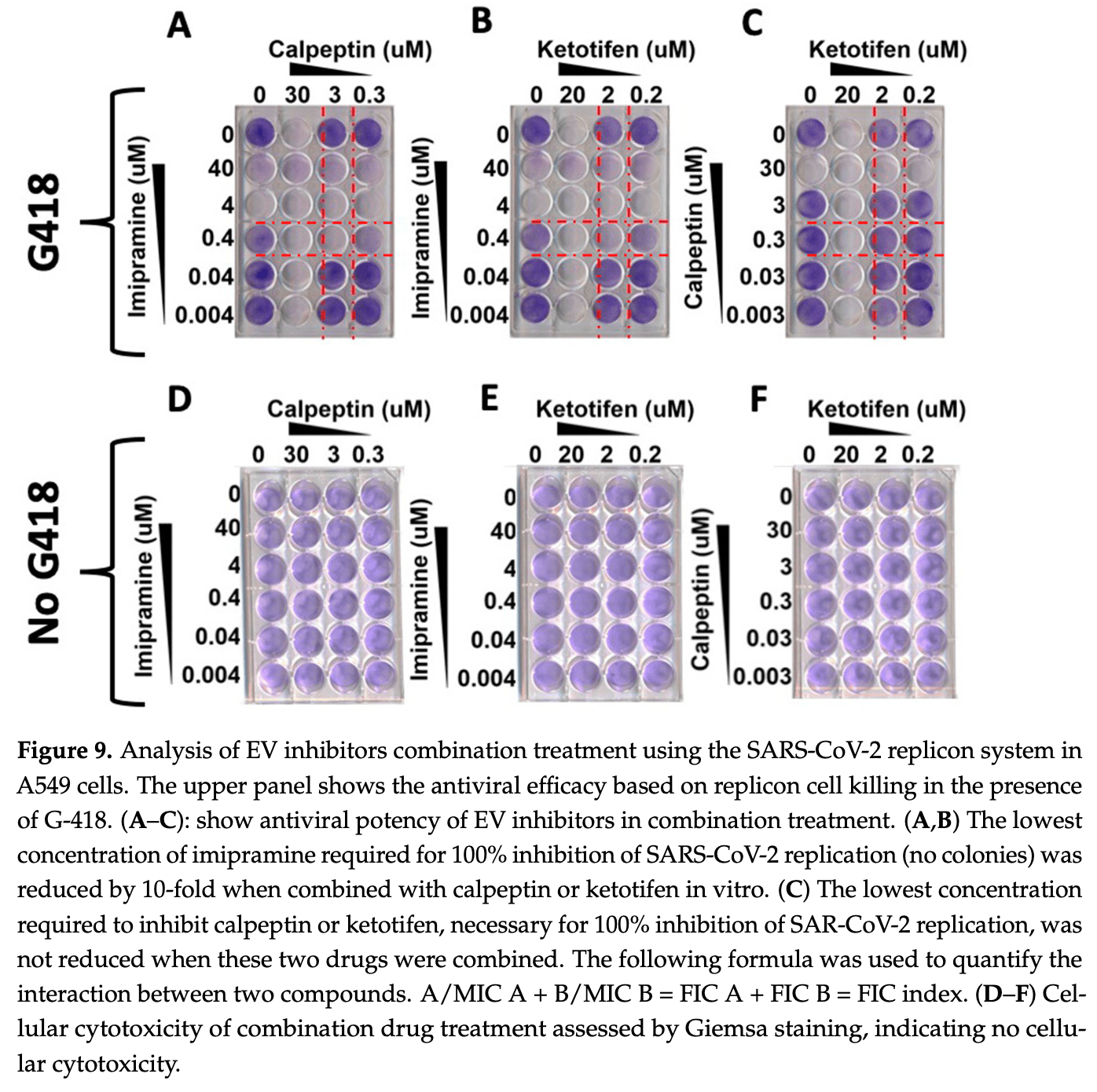
The concentration of extracellular vesicles (EVs) in the peripheral blood of COVID-19 patients is increased. Nevertheless, their potential role in the transmission of infection remains unclear. This study was performed to determine whether EVs produced by the sub-genomic replicon system developed in Baby Hamster Kidney (BHK-21) cells could transfer SARS-CoV-2 replicon RNA, leading to the establishment of a viral replication system in human cells. Purified EVs from the SARS-CoV-2 sub-genomic replicon cell line BHK-21 were cultured with a naive human cell line. The success of EV-mediated transfer of SARS-CoV-2 replicon RNA and its productive replication was assessed using G-418 selection, a luciferase assay, immunostaining, and Western blot. We found that the A549 cell line cultured with EVs isolated from SARS-CoV-2 BHK-21 replicon cells developed G-418-resistant cell colonies. SARS-COV-2 RNA replication in A549 cells was confirmed by nano luciferase, Nsp1 protein. SARS-CoV-2 RNA replication causes massive morphological changes. Treatment of cells with the FDA-approved Paxlovid demonstrated a dose-dependent inhibition of viral replication. We isolated two human epithelial cell lines (gastrointestinal and neuroblastoma) and one vascular endothelial cell line that stably support high-level replication of SARS-CoV-2 sub-genomic RNA. Viral elimination did not revert the abnormal cellular shape, vesicle accumulation, syncytia formation, or EV release. Our study's findings highlight the potential implications of EV-mediated transfer of replicon RNA to permissive cells. The replicon model is a valuable tool for studying virus-induced reversible and irreversible cellular reprogramming, as well as for testing novel therapeutic strategies for SARS-CoV-2.
Viruses 2026, 18, 145 Funding: This work was supported by funds derived from a Veterans Affairs Merit Review Grant (1101 BX004516-01A1), Bridge Funding from Tulane University Health Sciences Center, and a Seed Grant from the Louisiana Cancer Research Center. Nergiz Ekmen was supported by funds received from the Akdamar Fellowship Program, Department of Gastroenterology and Hepatology, Tulane University Health Science Center.
Institutional Review Board Statement: Not applicable. Informed Consent Statement: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest.
Abbreviations The following abbreviations are used in this manuscript: EVs, extracellular vesicles; BHK-21 Baby Hamster Kidney; SVEC, SV40 transformed mouse endothelial cell line; TEM, transmission electron microscopy; DMVs, double membrane vesicles; G-418, geneticin sulfate, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; MVs, microvesicles; CMs, convoluted membranes; DMSs, double-membrane spherules; sg mRNAs, sub-genomic mRNA transcription; BSL-3, biosafety level 3; IBC, Tulane Institutional Biosafety Committee; SLVHCS, Southeast Louisiana Veterans Health Care System; NTA, nanoparticle tracking analysis; LSM, light scattered mode; MFI, mean fluorescence intensity; TBS, tris-buffered saline; RT, room temperature; RLU, relative light units; Nano Luc, nano luciferase; A549, human lung alveolar epithelial cells; aSMase, sphingomyelinase; AFs, actin filaments; MTs,..
References
Akinosoglou, Schinas, Gogos, Oral antiviral treatment for COVID-19: A comprehensive review on nirmatrelvir/ritonavir, Viruses, doi:10.3390/v14112540
Aloi, Drago, Ruggieri, Cibella, Colombo et al., Extracellular vesicles and immunity: At the crossroads of cell communication, Int. J. Mol. Sci, doi:10.3390/ijms25021205
Aminpour, Hameroff, Tuszynski, How COVID-19 hijacks the cytoskeleton: Therapeutic implications, Life, doi:10.3390/life12060814
Aydin, Koksal, Reddy, Lin, Osman et al., Extracellular vesicle release promotes viral replication during persistent HCV infection, Cells, doi:10.3390/cells10050984
Barbosa, De Lima, Peachazepi Moraes, Borba-Junior, Huber et al., Angiopoietin2 is associated with coagulation activation and tissue factor expression in extracellular vesicles in COVID-19, Front. Med
Bartak, Chodkowski, Tymi Ńska, Ba Ńbura, Cymerys, Neurons cytoskeletal architecture remodeling during the replication cycle of mouse coronavirus MHV-JHM: A morphological in vitro study, BMC Vet. Res, doi:10.1186/s12917-023-03813-y
Bruggmann, Falcato, Dober, Helbling, Keiser et al., Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients, J. Viral Hepat, doi:10.1111/j.1365-2893.2008.01010.x
Buchrieser, Dufloo, Hubert, Monel, Planas et al., Syncytia formation by SARS-CoV-2-infected cells, EMBO J, doi:10.15252/embj.2020106267
Bussani, Schneider, Zentilin, Collesi, Ali et al., Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology, EBioMedicine, doi:10.1016/j.ebiom.2020.103104
Caldas, Carneiro, Higa, Monteiro, Da Silva et al., Ultrastructural analysis of SARS-CoV-2 interactions with the host cell via high resolution scanning electron microscopy, Sci. Rep, doi:10.1038/s41598-020-73162-5
Caldera-Crespo, Paidas, Roy, Schulman, Kenyon et al., Experimental models of COVID-19, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2021.792584
Chen, Lupan, Quek, Stanciu, Asaftei et al., A coronaviral pore-replicase complex links RNA synthesis and export from double-membrane vesicles, Sci. Adv, doi:10.1126/sciadv.adq9580
Chen, Turcinovic, Feng, Kenney, Chin et al., Cell culture systems for isolation of SARS-CoV-2 clinical isolates and generation of recombinant virus, Iscience, doi:10.1016/j.isci.2023.106634
Chen, Yuan, Hu, Zhu, Chen et al., SARS-CoV-2 immunity in animal models, Cell. Mol. Immunol, doi:10.1038/s41423-023-01122-w
Cooper, Van Heeswijk, Gallicano, Cameron, A review of low-dose ritonavir in protease inhibitor combination therapy, Clin. Infect. Dis, doi:10.1086/375233
Cortes-Galvez, Dangerfield, Metzner, Extracellular vesicles and their membranes: Exosomes vs. Virus-related particles, Membranes, doi:10.3390/membranes13040397
Cortese, Lee, Cerikan, Neufeldt, Oorschot et al., Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies, Cell Host Microbe, doi:10.1016/j.chom.2020.11.003
Dreux, Garaigorta, Boyd, Décembre, Chung et al., Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity, Cell Host Microbe, doi:10.1016/j.chom.2012.08.010
Eagling, Back, Barry, Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir, Br. J. Clin. Pharmacol, doi:10.1046/j.1365-2125.1997.00644.x
Fafi-Kremer, Fofana, Soulier, Carolla, Meuleman et al., Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation, J. Exp. Med, doi:10.1084/jem.20090766
Feng, Hensley, Mcknight, Hu, Madden et al., A pathogenic picornavirus acquires an envelope by hijacking cellular membranes, Nature, doi:10.1038/nature12029
Ferrandez-Pujante, Pons-Fuster, López-Jornet, Efficacy of photobiomodulation in reducing symptomatology and improving the quality of life in patients with xerostomia and hyposalivation: A randomized controlled trial, J. Clin. Med, doi:10.3390/jcm11123414
González, Dias, Reis, Villalba, Vazquez et al., Effective RNA extraction with readily available reagents: A comparative analysis of alternative protocols, J. Virol. Methods, doi:10.1016/j.jviromet.2025.115242
Grünvogel, Colasanti, Lee, Klöss, Belouzard et al., Secretion of hepatitis C virus replication intermediates reduces activation of toll-like receptor 3 in hepatocytes, Gastroenterology, doi:10.1053/j.gastro.2018.03.020
He, Quan, Xu, Rodriguez, Goh et al., Generation of SARS-CoV-2 reporter replicon for high-throughput antiviral screening and testing, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2025866118
Hernández-Díazcouder, Díaz-Godínez, Carrero, Extracellular vesicles in COVID-19 prognosis, treatment, and vaccination: An update, Appl. Microbiol. Biotechnol, doi:10.1007/s00253-023-12468-6
Huang, Lian, Song, Ma, Lian et al., Clinical features of severe patients infected with 2019 novel coronavirus: A systematic review and meta-analysis, Ann. Transl. Med, doi:10.21037/atm-20-2124
Huang, Wang, Zhong, Zhang, Zhang et al., Molecular architecture of coronavirus doublemembrane vesicle pore complex, Nature, doi:10.1038/s41586-024-07817-y
Jackson Cullison, Flemming, Karagoz, Wermuth, Mahoney, Mechanisms of extracellular vesicle uptake and implications for the design of cancer therapeutics, J. Extracell. Biol, doi:10.1002/jex2.70017
Jacob, Lambour, Swinyard, Zerbib, Diouf et al., Annexin-V positive extracellular vesicles level is increased in severe COVID-19 disease, Front. Med, doi:10.3389/fmed.2023.1186122
Jochems, Garssen, Van Keulen, Masereeuw, Jeurink, Evaluating human intestinal cell lines for studying dietary protein absorption, Nutrients, doi:10.3390/nu10030322
Kim, Lee, Baek, Dissecting exosome inhibitors: Therapeutic insights into small-molecule chemicals against cancer, Exp. Mol. Med, doi:10.1038/s12276-022-00898-7
Klein, Cortese, Winter, Wachsmuth-Melm, Neufeldt et al., SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography, Nat. Commun, doi:10.1038/s41467-020-19619-7
Kloc, Uosef, Wosik, Kubiak, Ghobrial, Virus interactions with the actin cytoskeleton-What we know and do not know about SARS-CoV-2, Arch. Virol, doi:10.1007/s00705-022-05366-1
Koksal, Ekmen, Aydin, Nunez, Sandow et al., A Single-Step Immunocapture Assay to Quantify HCC Exosomes Using the Highly Sensitive Fluorescence Nanoparticle-Tracking Analysis, J. Hepatocell. Carcinoma, doi:10.2147/JHC.S423043
Kosanović, Milutinović, Goč, Mitić, Janković, Ion-exchange chromatography purification of extracellular vesicles, Biotechniques, doi:10.2144/000114575
Lee, The complex role of extracellular vesicles in HIV infection, BMB Rep, doi:10.5483/BMBRep.2023-0073
Li, Li, Liu, Zha, Extracellular vesicles regulate the transmission of insulin resistance and redefine noncommunicable diseases, Front. Mol. Biosci, doi:10.3389/fmolb.2022.1024786
Liu, Chou, Wu, Luan, Wang, Stable cell clones harboring self-replicating SARS-CoV-2 RNAs for drug screen, J. Virol, doi:10.1128/jvi.02216-21
Lowery, Kuczmarski, Herrmann, Goldman, Intermediate filaments play a pivotal role in regulating cell architecture and function, J. Biol. Chem, doi:10.1074/jbc.R115.640359
Ludwig, De Miroschedji, Doeppner, Börger, Ruesing et al., Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales, J. Extracell. Vesicles, doi:10.1080/20013078.2018.1528109
Luna, Thao, Le Pen, Yu, Hoffmann et al., Replication and single-cycle delivery of SARS-CoV-2 replicons, Science, doi:10.1126/science.abj8430
Malone, Urakova, Snijder, Campbell, Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00432-z
Martin, Ligat, Malnou, The Yin and the Yang of extracellular vesicles during viral infections, Biomed. J
Martins, Alves, Extracellular vesicles in viral infections: Two sides of the same coin? Front, Cell. Infect. Microbiol, doi:10.3389/fcimb.2020.593170
Masaki, Ahmed, Ishida, Mikami, Funabashi et al., Chromatographic purification of small extracellular vesicles using an affinity column for phospholipid membranes, Biotechnol. Lett, doi:10.1007/s10529-023-03430-7
Masciopinto, Giovani, Campagnoli, Galli-Stampino, Colombatto et al., Association of hepatitis C virus envelope proteins with exosomes, Eur. J. Immunol, doi:10.1002/eji.200424887
Moulin, Crupi, Ilkow, Bell, Boulton, Extracellular vesicles and viruses: Two intertwined entities, Int. J. Mol. Sci, doi:10.3390/ijms24021036
Mulcahy, Pink, Carter, Routes and mechanisms of extracellular vesicle uptake, J. Extracell. Vesicles, doi:10.3402/jev.v3.24641
Naqvi, Fatima, Mohammad, Fatima, Singh et al., Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach, Biochim. Biophys. Acta (BBA)-Mol. Basis Dis, doi:10.1016/j.bbadis.2020.165878
Nguyen, Falzarano, Gerdts, Liu, Construction of a noninfectious SARS-CoV-2 replicon for antiviral-drug testing and gene function studies, J. Virol, doi:10.1128/JVI.00687-21
Odilov, Volkov, Abdullaev, Gasanova, Lipina et al., COVID-19: Multiorgan dissemination of SARS-CoV-2 is driven by pulmonary factors, Viruses, doi:10.3390/v14010039
Qian, Zang, Zhang, Zheng, Qiu et al., Circulating extracellular vesicles from severe COVID-19 patients induce lung inflammation, mSphere, doi:10.1128/msphere.00764-24
Ramakrishnaiah, Thumann, Fofana, Habersetzer, Pan et al., Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7. 5 cells, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1221899110
Roingeard, Eymieux, Burlaud-Gaillard, Hourioux, Patient et al., The double-membrane vesicle (DMV): A virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses, Cell. Mol. Life Sci, doi:10.1007/s00018-022-04469-x
Sabatke, Rossi, Ramirez, Interaction vesicles as emerging mediators of host-pathogen molecular crosstalk and their implications for infection dynamics, FEBS Lett, doi:10.1002/1873-3468.70055
Su, Wu, Xie, Zheng, Chen et al., A Review of Extracellular Vesicles in COVID-19 Diagnosis, Treatment, and Prevention, Adv. Sci, doi:10.1002/advs.202206095
Subudhi, Rooge, Bihari, Thangariyal, Goswami et al., Prolonged existence of SARS-CoV-2 RNAs in the extracellular vesicles of respiratory specimens from patients with negative reverse transcription-polymerase chain reaction, Liver Res
Swain, Merida, Rubio, Bracquemond, Neyret et al., F-actin nanostructures rearrangements and regulation are essential for SARS-CoV-2 particle production in host pulmonary cells, iScience, doi:10.1016/j.isci.2023.107384
Tamai, Shiina, Tanaka, Nakano, Yamamoto et al., Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway, Virology, doi:10.1016/j.virol.2011.11.009
Thierry, Usher, Sanchez, Turner, Venter et al., Circulating Microclots Are Structurally Associated With Neutrophil Extracellular Traps and Their Amounts Are Elevated in Long COVID Patients, J. Med. Virol, doi:10.1002/jmv.70613
Théry, Zitvogel, Amigorena, Exosomes: Composition, biogenesis and function, Nat. Rev. Immunol, doi:10.1038/nri855
Timpe, Stamataki, Jennings, Hu, Farquhar et al., Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies, Hepatology, doi:10.1002/hep.21959
Vangeel, Chiu, De Jonghe, Maes, Slechten et al., Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antivir. Res, doi:10.1016/j.antiviral.2022.105252
Wang, Zhang, Liang, Liu, Deng et al., Extracellular vesicles originating from autophagy mediate an antibody-resistant spread of classical swine fever virus in cell culture, Autophagy, doi:10.1080/15548627.2021.1987673
Wen, Zhang, Lin, Shi, Jiu, Cytoskeleton-A crucial key in host cell for coronavirus infection, J. Mol. Cell Biol, doi:10.1093/jmcb/mjaa042
Wolff, Melia, Snijder, Bárcena, Double-membrane vesicles as platforms for viral replication, Trends Microbiol, doi:10.1016/j.tim.2020.05.009
Xia, Pan, Luo, Shen, Li et al., Extracellular vesicles mediate antibodyresistant transmission of SARS-CoV-2, Cell Discov, doi:10.1038/s41421-022-00510-2
Xie, Muruato, Lokugamage, Narayanan, Zhang et al., An infectious cDNA clone of SARS-CoV-2, Cell Host Microbe, doi:10.1016/j.chom.2020.04.004
Zhao, Zhang, Liang, Lei, Han et al., SARS-CoV-2 polyprotein expression and the induction of double-membrane vesicles, J. Virol, doi:10.1128/jvi.01385-25
Zhou, Gammeltoft, Ryberg, Pham, Tjørnelund et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Sci. Adv, doi:10.1126/sciadv.add7197
Zhu, Zhang, Wang, Li, Yang et al., A novel coronavirus from patients with pneumonia in China, N. Engl. J. Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3390/v18010145",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v18010145",
"abstract": "<jats:p>The concentration of extracellular vesicles (EVs) in the peripheral blood of COVID-19 patients is increased. Nevertheless, their potential role in the transmission of infection remains unclear. This study was performed to determine whether EVs produced by the sub-genomic replicon system developed in Baby Hamster Kidney (BHK-21) cells could transfer SARS-CoV-2 replicon RNA, leading to the establishment of a viral replication system in human cells. Purified EVs from the SARS-CoV-2 sub-genomic replicon cell line BHK-21 were cultured with a naive human cell line. The success of EV-mediated transfer of SARS-CoV-2 replicon RNA and its productive replication was assessed using G-418 selection, a luciferase assay, immunostaining, and Western blot. We found that the A549 cell line cultured with EVs isolated from SARS-CoV-2 BHK-21 replicon cells developed G-418-resistant cell colonies. SARS-COV-2 RNA replication in A549 cells was confirmed by nano luciferase, Nsp1 protein. SARS-CoV-2 RNA replication causes massive morphological changes. Treatment of cells with the FDA-approved Paxlovid demonstrated a dose-dependent inhibition of viral replication. We isolated two human epithelial cell lines (gastrointestinal and neuroblastoma) and one vascular endothelial cell line that stably support high-level replication of SARS-CoV-2 sub-genomic RNA. Viral elimination did not revert the abnormal cellular shape, vesicle accumulation, syncytia formation, or EV release. Our study’s findings highlight the potential implications of EV-mediated transfer of replicon RNA to permissive cells. The replicon model is a valuable tool for studying virus-induced reversible and irreversible cellular reprogramming, as well as for testing novel therapeutic strategies for SARS-CoV-2.</jats:p>",
"alternative-id": [
"v18010145"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-7921-3169",
"affiliation": [
{
"name": "Division of Gastroenterology and Hepatology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA"
}
],
"authenticated-orcid": false,
"family": "Ekmen",
"given": "Nergiz",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-5693-5951",
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine, Tulane University Health Sciences Center, 1430 Tulane Avenue, New Orleans, LA 70112, USA"
}
],
"authenticated-orcid": false,
"family": "Koksal",
"given": "Ali Riza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine, Tulane University Health Sciences Center, 1430 Tulane Avenue, New Orleans, LA 70112, USA"
}
],
"family": "Lin",
"given": "Dong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine, Tulane University Health Sciences Center, 1430 Tulane Avenue, New Orleans, LA 70112, USA"
}
],
"family": "Tian",
"given": "Di",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6483-6259",
"affiliation": [
{
"name": "Department of Gastroenterology and Hepatology, Institute of Translational Research, Ochsner Health, New Orleans, LA 70121, USA"
}
],
"authenticated-orcid": false,
"family": "Thevenot",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Gastroenterology and Hepatology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA"
}
],
"family": "Glover",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Gastroenterology and Hepatology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA"
},
{
"name": "Department of Pathology and Laboratory Medicine, Tulane University Health Sciences Center, 1430 Tulane Avenue, New Orleans, LA 70112, USA"
},
{
"name": "Southeast Louisiana Veterans Health Care System, 2400 Canal Street, New Orleans, LA 70119, USA"
}
],
"family": "Dash",
"given": "Srikanta",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
23
]
],
"date-time": "2026-01-23T10:41:34Z",
"timestamp": 1769164894000
},
"deposited": {
"date-parts": [
[
2026,
1,
23
]
],
"date-time": "2026-01-23T10:47:26Z",
"timestamp": 1769165246000
},
"funder": [
{
"award": [
"1101 BX004516-01A1"
],
"award-info": [
{
"award-number": [
"1101 BX004516-01A1"
]
}
],
"name": "Veterans Affairs Merit Review"
},
{
"name": "Tulane University Health Sciences Center"
},
{
"name": "Seed Grant from the Louisiana Cancer Research Center"
},
{
"name": "Akdamar Fellowship Program"
},
{
"name": "Department of Gastroenterology and Hepatology, Tulane University Health Science Center"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
24
]
],
"date-time": "2026-01-24T20:10:42Z",
"timestamp": 1769285442680,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2026,
1,
22
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2026,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
22
]
],
"date-time": "2026-01-22T00:00:00Z",
"timestamp": 1769040000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/18/1/145/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "145",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2026,
1,
22
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
22
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N. Engl. J. Med.",
"key": "ref_1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.21037/atm-20-2124",
"article-title": "Clinical features of severe patients infected with 2019 novel coronavirus: A systematic review and meta-analysis",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "576",
"journal-title": "Ann. Transl. Med.",
"key": "ref_2",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/v14010039",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Odilov, A., Volkov, A., Abdullaev, A., Gasanova, T., Lipina, T., and Babichenko, I. (2021). COVID-19: Multiorgan dissemination of SARS-CoV-2 is driven by pulmonary factors. Viruses, 14."
},
{
"DOI": "10.1038/nri855",
"article-title": "Exosomes: Composition, biogenesis and function",
"author": "Zitvogel",
"doi-asserted-by": "crossref",
"first-page": "569",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_4",
"volume": "2",
"year": "2002"
},
{
"DOI": "10.3389/fmolb.2022.1024786",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Li, B., Li, W., Liu, T., and Zha, L. (2023). Extracellular vesicles regulate the transmission of insulin resistance and redefine noncommunicable diseases. Front. Mol. Biosci., 9."
},
{
"DOI": "10.3390/ijms25021205",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Aloi, N., Drago, G., Ruggieri, S., Cibella, F., Colombo, P., and Longo, V. (2024). Extracellular vesicles and immunity: At the crossroads of cell communication. Int. J. Mol. Sci., 25."
},
{
"DOI": "10.1080/15548627.2021.1987673",
"article-title": "Extracellular vesicles originating from autophagy mediate an antibody-resistant spread of classical swine fever virus in cell culture",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1433",
"journal-title": "Autophagy",
"key": "ref_7",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.3390/ijms24021036",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Moulin, C., Crupi, M.J., Ilkow, C.S., Bell, J.C., and Boulton, S. (2023). Extracellular vesicles and viruses: Two intertwined entities. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.3389/fcimb.2020.593170",
"doi-asserted-by": "crossref",
"key": "ref_9",
"unstructured": "Martins, S.d.T., and Alves, L.R. (2020). Extracellular vesicles in viral infections: Two sides of the same coin?. Front. Cell. Infect. Microbiol., 10."
},
{
"DOI": "10.1016/j.bj.2023.100659",
"article-title": "The Yin and the Yang of extracellular vesicles during viral infections",
"author": "Martin",
"doi-asserted-by": "crossref",
"first-page": "100659",
"journal-title": "Biomed. J.",
"key": "ref_10",
"volume": "47",
"year": "2024"
},
{
"DOI": "10.3390/cells10050984",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Aydin, Y., Koksal, A.R., Reddy, V., Lin, D., Osman, H., Heidari, Z., Rhadhi, S.M., Wimley, W.C., Parsi, M.A., and Dash, S. (2021). Extracellular vesicle release promotes viral replication during persistent HCV infection. Cells, 10."
},
{
"DOI": "10.1038/nature12029",
"article-title": "A pathogenic picornavirus acquires an envelope by hijacking cellular membranes",
"author": "Feng",
"doi-asserted-by": "crossref",
"first-page": "367",
"journal-title": "Nature",
"key": "ref_12",
"volume": "496",
"year": "2013"
},
{
"DOI": "10.1073/pnas.1221899110",
"article-title": "Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7. 5 cells",
"author": "Ramakrishnaiah",
"doi-asserted-by": "crossref",
"first-page": "13109",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_13",
"volume": "110",
"year": "2013"
},
{
"DOI": "10.1002/hep.21959",
"article-title": "Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies",
"author": "Timpe",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Hepatology",
"key": "ref_14",
"volume": "47",
"year": "2008"
},
{
"DOI": "10.1111/j.1365-2893.2008.01010.x",
"article-title": "Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients",
"author": "Bruggmann",
"doi-asserted-by": "crossref",
"first-page": "747",
"journal-title": "J. Viral Hepat.",
"key": "ref_15",
"volume": "15",
"year": "2008"
},
{
"DOI": "10.1084/jem.20090766",
"article-title": "Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation",
"author": "Fofana",
"doi-asserted-by": "crossref",
"first-page": "2019",
"journal-title": "J. Exp. Med.",
"key": "ref_16",
"volume": "207",
"year": "2010"
},
{
"DOI": "10.1002/eji.200424887",
"article-title": "Association of hepatitis C virus envelope proteins with exosomes",
"author": "Masciopinto",
"doi-asserted-by": "crossref",
"first-page": "2834",
"journal-title": "Eur. J. Immunol.",
"key": "ref_17",
"volume": "34",
"year": "2004"
},
{
"DOI": "10.1016/j.virol.2011.11.009",
"article-title": "Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway",
"author": "Tamai",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "Virology",
"key": "ref_18",
"volume": "422",
"year": "2012"
},
{
"DOI": "10.1016/j.chom.2012.08.010",
"article-title": "Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity",
"author": "Dreux",
"doi-asserted-by": "crossref",
"first-page": "558",
"journal-title": "Cell Host Microbe",
"key": "ref_19",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.5483/BMBRep.2023-0073",
"article-title": "The complex role of extracellular vesicles in HIV infection",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "335",
"journal-title": "BMB Rep.",
"key": "ref_20",
"volume": "56",
"year": "2023"
},
{
"DOI": "10.3389/fmed.2023.1186122",
"doi-asserted-by": "crossref",
"key": "ref_21",
"unstructured": "Jacob, V., Lambour, A., Swinyard, B., Zerbib, Y., Diouf, M., Soudet, S., Brochot, E., Six, I., Maizel, J., and Slama, M. (2023). Annexin-V positive extracellular vesicles level is increased in severe COVID-19 disease. Front. Med., 10."
},
{
"DOI": "10.1016/j.bbadis.2020.165878",
"article-title": "Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach",
"author": "Naqvi",
"doi-asserted-by": "crossref",
"first-page": "165878",
"journal-title": "Biochim. Biophys. Acta (BBA)-Mol. Basis Dis.",
"key": "ref_22",
"volume": "1866",
"year": "2020"
},
{
"DOI": "10.1038/s41580-021-00432-z",
"article-title": "Structures and functions of coronavirus replication–transcription complexes and their relevance for SARS-CoV-2 drug design",
"author": "Malone",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_23",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1007/s00018-022-04469-x",
"article-title": "The double-membrane vesicle (DMV): A virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses",
"author": "Roingeard",
"doi-asserted-by": "crossref",
"first-page": "425",
"journal-title": "Cell. Mol. Life Sci.",
"key": "ref_24",
"volume": "79",
"year": "2022"
},
{
"DOI": "10.1016/j.tim.2020.05.009",
"article-title": "Double-membrane vesicles as platforms for viral replication",
"author": "Wolff",
"doi-asserted-by": "crossref",
"first-page": "1022",
"journal-title": "Trends Microbiol.",
"key": "ref_25",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1002/advs.202206095",
"article-title": "A Review of Extracellular Vesicles in COVID-19 Diagnosis, Treatment, and Prevention",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "2206095",
"journal-title": "Adv. Sci.",
"key": "ref_26",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1007/s00253-023-12468-6",
"article-title": "Extracellular vesicles in COVID-19 prognosis, treatment, and vaccination: An update",
"author": "Carrero",
"doi-asserted-by": "crossref",
"first-page": "2131",
"journal-title": "Appl. Microbiol. Biotechnol.",
"key": "ref_27",
"volume": "107",
"year": "2023"
},
{
"DOI": "10.1128/jvi.02216-21",
"article-title": "Stable cell clones harboring self-replicating SARS-CoV-2 RNAs for drug screen",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "e02216-02221",
"journal-title": "J. Virol.",
"key": "ref_28",
"volume": "96",
"year": "2022"
},
{
"DOI": "10.2147/JHC.S423043",
"article-title": "A Single-Step Immunocapture Assay to Quantify HCC Exosomes Using the Highly Sensitive Fluorescence Nanoparticle-Tracking Analysis",
"author": "Koksal",
"doi-asserted-by": "crossref",
"first-page": "1935",
"journal-title": "J. Hepatocell. Carcinoma",
"key": "ref_29",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1080/20013078.2018.1528109",
"article-title": "Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales",
"author": "Ludwig",
"doi-asserted-by": "crossref",
"first-page": "1528109",
"journal-title": "J. Extracell. Vesicles",
"key": "ref_30",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1038/s41467-020-19619-7",
"article-title": "SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography",
"author": "Klein",
"doi-asserted-by": "crossref",
"first-page": "5885",
"journal-title": "Nat. Commun.",
"key": "ref_31",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.livres.2023.09.004",
"article-title": "Prolonged existence of SARS-CoV-2 RNAs in the extracellular vesicles of respiratory specimens from patients with negative reverse transcription-polymerase chain reaction",
"author": "Subudhi",
"doi-asserted-by": "crossref",
"first-page": "228",
"journal-title": "Liver Res.",
"key": "ref_32",
"volume": "7",
"year": "2023"
},
{
"DOI": "10.1038/s41421-022-00510-2",
"article-title": "Extracellular vesicles mediate antibody-resistant transmission of SARS-CoV-2",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Cell Discov.",
"key": "ref_33",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1093/jmcb/mjaa042",
"article-title": "Cytoskeleton—A crucial key in host cell for coronavirus infection",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "968",
"journal-title": "J. Mol. Cell Biol.",
"key": "ref_34",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/membranes13040397",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Cortes-Galvez, D., Dangerfield, J.A., and Metzner, C. (2023). Extracellular vesicles and their membranes: Exosomes vs. Virus-related particles. Membranes, 13."
},
{
"DOI": "10.2144/000114575",
"article-title": "Ion-exchange chromatography purification of extracellular vesicles",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "Biotechniques",
"key": "ref_36",
"volume": "63",
"year": "2017"
},
{
"DOI": "10.1007/s10529-023-03430-7",
"article-title": "Chromatographic purification of small extracellular vesicles using an affinity column for phospholipid membranes",
"author": "Masaki",
"doi-asserted-by": "crossref",
"first-page": "1457",
"journal-title": "Biotechnol. Lett.",
"key": "ref_37",
"volume": "45",
"year": "2023"
},
{
"DOI": "10.1016/j.jviromet.2025.115242",
"article-title": "Effective RNA extraction with readily available reagents: A comparative analysis of alternative protocols",
"author": "Dias",
"doi-asserted-by": "crossref",
"first-page": "115242",
"journal-title": "J. Virol. Methods",
"key": "ref_38",
"volume": "338",
"year": "2025"
},
{
"DOI": "10.1038/s12276-022-00898-7",
"article-title": "Dissecting exosome inhibitors: Therapeutic insights into small-molecule chemicals against cancer",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "1833",
"journal-title": "Exp. Mol. Med.",
"key": "ref_39",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1074/jbc.R115.640359",
"article-title": "Intermediate filaments play a pivotal role in regulating cell architecture and function",
"author": "Lowery",
"doi-asserted-by": "crossref",
"first-page": "17145",
"journal-title": "J. Biol. Chem.",
"key": "ref_40",
"volume": "290",
"year": "2015"
},
{
"DOI": "10.1038/s41423-023-01122-w",
"article-title": "SARS-CoV-2 immunity in animal models",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "119",
"journal-title": "Cell. Mol. Immunol.",
"key": "ref_41",
"volume": "21",
"year": "2024"
},
{
"DOI": "10.15252/embj.2020106267",
"article-title": "Syncytia formation by SARS-CoV-2-infected cells",
"author": "Buchrieser",
"doi-asserted-by": "crossref",
"first-page": "e106267",
"journal-title": "EMBO J.",
"key": "ref_42",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2020.103104",
"article-title": "Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology",
"author": "Bussani",
"doi-asserted-by": "crossref",
"first-page": "103104",
"journal-title": "EBioMedicine",
"key": "ref_43",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.1126/sciadv.add7197",
"article-title": "Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "eadd7197",
"journal-title": "Sci. Adv.",
"key": "ref_44",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"article-title": "Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern",
"author": "Vangeel",
"doi-asserted-by": "crossref",
"first-page": "105252",
"journal-title": "Antivir. Res.",
"key": "ref_45",
"volume": "198",
"year": "2022"
},
{
"DOI": "10.3390/v14112540",
"doi-asserted-by": "crossref",
"key": "ref_46",
"unstructured": "Akinosoglou, K., Schinas, G., and Gogos, C. (2022). Oral antiviral treatment for COVID-19: A comprehensive review on nirmatrelvir/ritonavir. Viruses, 14."
},
{
"DOI": "10.1086/375233",
"article-title": "A review of low-dose ritonavir in protease inhibitor combination therapy",
"author": "Cooper",
"doi-asserted-by": "crossref",
"first-page": "1585",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_47",
"volume": "36",
"year": "2003"
},
{
"DOI": "10.1046/j.1365-2125.1997.00644.x",
"article-title": "Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir",
"author": "Eagling",
"doi-asserted-by": "crossref",
"first-page": "190",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_48",
"volume": "44",
"year": "1997"
},
{
"DOI": "10.1016/j.isci.2023.106634",
"article-title": "Cell culture systems for isolation of SARS-CoV-2 clinical isolates and generation of recombinant virus",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "106634",
"journal-title": "Iscience",
"key": "ref_49",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1186/s12917-023-03813-y",
"doi-asserted-by": "crossref",
"key": "ref_50",
"unstructured": "Bartak, M., Bąska, P., Chodkowski, M., Tymińska, B., Bańbura, M.W., and Cymerys, J. (2024). Neurons cytoskeletal architecture remodeling during the replication cycle of mouse coronavirus MHV-JHM: A morphological in vitro study. BMC Vet. Res., 20."
},
{
"DOI": "10.3390/jcm11123414",
"doi-asserted-by": "crossref",
"key": "ref_51",
"unstructured": "Ferrandez-Pujante, A., Pons-Fuster, E., and López-Jornet, P. (2022). Efficacy of photobiomodulation in reducing symptomatology and improving the quality of life in patients with xerostomia and hyposalivation: A randomized controlled trial. J. Clin. Med., 11."
},
{
"DOI": "10.3390/nu10030322",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Jochems, P.G., Garssen, J., Van Keulen, A.M., Masereeuw, R., and Jeurink, P.V. (2018). Evaluating human intestinal cell lines for studying dietary protein absorption. Nutrients, 10."
},
{
"DOI": "10.1002/jmv.70613",
"article-title": "Circulating Microclots Are Structurally Associated With Neutrophil Extracellular Traps and Their Amounts Are Elevated in Long COVID Patients",
"author": "Thierry",
"doi-asserted-by": "crossref",
"first-page": "e70613",
"journal-title": "J. Med. Virol.",
"key": "ref_53",
"volume": "97",
"year": "2025"
},
{
"DOI": "10.1128/msphere.00764-24",
"article-title": "Circulating extracellular vesicles from severe COVID-19 patients induce lung inflammation",
"author": "Qian",
"doi-asserted-by": "crossref",
"first-page": "e00764-00724",
"journal-title": "mSphere",
"key": "ref_54",
"volume": "9",
"year": "2024"
},
{
"DOI": "10.3389/fmed.2024.1367544",
"doi-asserted-by": "crossref",
"key": "ref_55",
"unstructured": "Barbosa, M.S., de Lima, F., Peachazepi Moraes, C.R., Borba-Junior, I.T., Huber, S.C., Santos, I., Bombassaro, B., Dertkigil, S.S.J., Ilich, A., and Key, N.S. (2024). Angiopoietin2 is associated with coagulation activation and tissue factor expression in extracellular vesicles in COVID-19. Front. Med., 11."
},
{
"DOI": "10.3389/fcimb.2021.792584",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Caldera-Crespo, L.A., Paidas, M.J., Roy, S., Schulman, C.I., Kenyon, N.S., Daunert, S., and Jayakumar, A.R. (2022). Experimental models of COVID-19. Front. Cell. Infect. Microbiol., 11."
},
{
"DOI": "10.1016/j.chom.2020.04.004",
"article-title": "An infectious cDNA clone of SARS-CoV-2",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "841",
"journal-title": "Cell Host Microbe",
"key": "ref_57",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2025866118",
"article-title": "Generation of SARS-CoV-2 reporter replicon for high-throughput antiviral screening and testing",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "e2025866118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_58",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1126/science.abj8430",
"article-title": "Replication and single-cycle delivery of SARS-CoV-2 replicons",
"author": "Luna",
"doi-asserted-by": "crossref",
"first-page": "1099",
"journal-title": "Science",
"key": "ref_59",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1128/JVI.00687-21",
"doi-asserted-by": "crossref",
"key": "ref_60",
"unstructured": "Nguyen, H.T., Falzarano, D., Gerdts, V., and Liu, Q. (2021). Construction of a noninfectious SARS-CoV-2 replicon for antiviral-drug testing and gene function studies. J. Virol., 95."
},
{
"DOI": "10.3402/jev.v3.24641",
"article-title": "Routes and mechanisms of extracellular vesicle uptake",
"author": "Mulcahy",
"doi-asserted-by": "crossref",
"first-page": "24641",
"journal-title": "J. Extracell. Vesicles",
"key": "ref_61",
"volume": "3",
"year": "2014"
},
{
"DOI": "10.1002/jex2.70017",
"article-title": "Mechanisms of extracellular vesicle uptake and implications for the design of cancer therapeutics",
"author": "Flemming",
"doi-asserted-by": "crossref",
"first-page": "e70017",
"journal-title": "J. Extracell. Biol.",
"key": "ref_62",
"volume": "3",
"year": "2024"
},
{
"DOI": "10.1002/1873-3468.70055",
"article-title": "Interaction vesicles as emerging mediators of host-pathogen molecular crosstalk and their implications for infection dynamics",
"author": "Sabatke",
"doi-asserted-by": "crossref",
"first-page": "2439",
"journal-title": "FEBS Lett.",
"key": "ref_63",
"volume": "599",
"year": "2025"
},
{
"DOI": "10.1038/s41598-020-73162-5",
"article-title": "Ultrastructural analysis of SARS-CoV-2 interactions with the host cell via high resolution scanning electron microscopy",
"author": "Caldas",
"doi-asserted-by": "crossref",
"first-page": "16099",
"journal-title": "Sci. Rep.",
"key": "ref_64",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1007/s00705-022-05366-1",
"article-title": "Virus interactions with the actin cytoskeleton—What we know and do not know about SARS-CoV-2",
"author": "Kloc",
"doi-asserted-by": "crossref",
"first-page": "737",
"journal-title": "Arch. Virol.",
"key": "ref_65",
"volume": "167",
"year": "2022"
},
{
"DOI": "10.3390/life12060814",
"doi-asserted-by": "crossref",
"key": "ref_66",
"unstructured": "Aminpour, M., Hameroff, S., and Tuszynski, J.A. (2022). How COVID-19 hijacks the cytoskeleton: Therapeutic implications. Life, 12."
},
{
"DOI": "10.1016/j.chom.2020.11.003",
"article-title": "Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies",
"author": "Cortese",
"doi-asserted-by": "crossref",
"first-page": "853",
"journal-title": "Cell Host Microbe",
"key": "ref_67",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2023.107384",
"article-title": "F-actin nanostructures rearrangements and regulation are essential for SARS-CoV-2 particle production in host pulmonary cells",
"author": "Swain",
"doi-asserted-by": "crossref",
"first-page": "107384",
"journal-title": "iScience",
"key": "ref_68",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1038/s41586-024-07817-y",
"article-title": "Molecular architecture of coronavirus double-membrane vesicle pore complex",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "224",
"journal-title": "Nature",
"key": "ref_69",
"volume": "633",
"year": "2024"
},
{
"DOI": "10.1128/jvi.01385-25",
"article-title": "SARS-CoV-2 polyprotein expression and the induction of double-membrane vesicles",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "e01385-25",
"journal-title": "J. Virol.",
"key": "ref_70",
"volume": "99",
"year": "2025"
},
{
"DOI": "10.1126/sciadv.adq9580",
"article-title": "A coronaviral pore-replicase complex links RNA synthesis and export from double-membrane vesicles",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "eadq9580",
"journal-title": "Sci. Adv.",
"key": "ref_71",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1053/j.gastro.2018.03.020",
"article-title": "Secretion of hepatitis C virus replication intermediates reduces activation of toll-like receptor 3 in hepatocytes",
"author": "Colasanti",
"doi-asserted-by": "crossref",
"first-page": "2237",
"journal-title": "Gastroenterology",
"key": "ref_72",
"volume": "154",
"year": "2018"
}
],
"reference-count": 72,
"references-count": 72,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/18/1/145"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Virion-Independent Extracellular Vesicle (EV)-Dependent Transmission of SARS-CoV-2 as a Potential New Mechanism of Viral RNA Spread in Human Cells",
"type": "journal-article",
"update-policy": "https://doi.org/10.3390/mdpi_crossmark_policy",
"volume": "18"
}