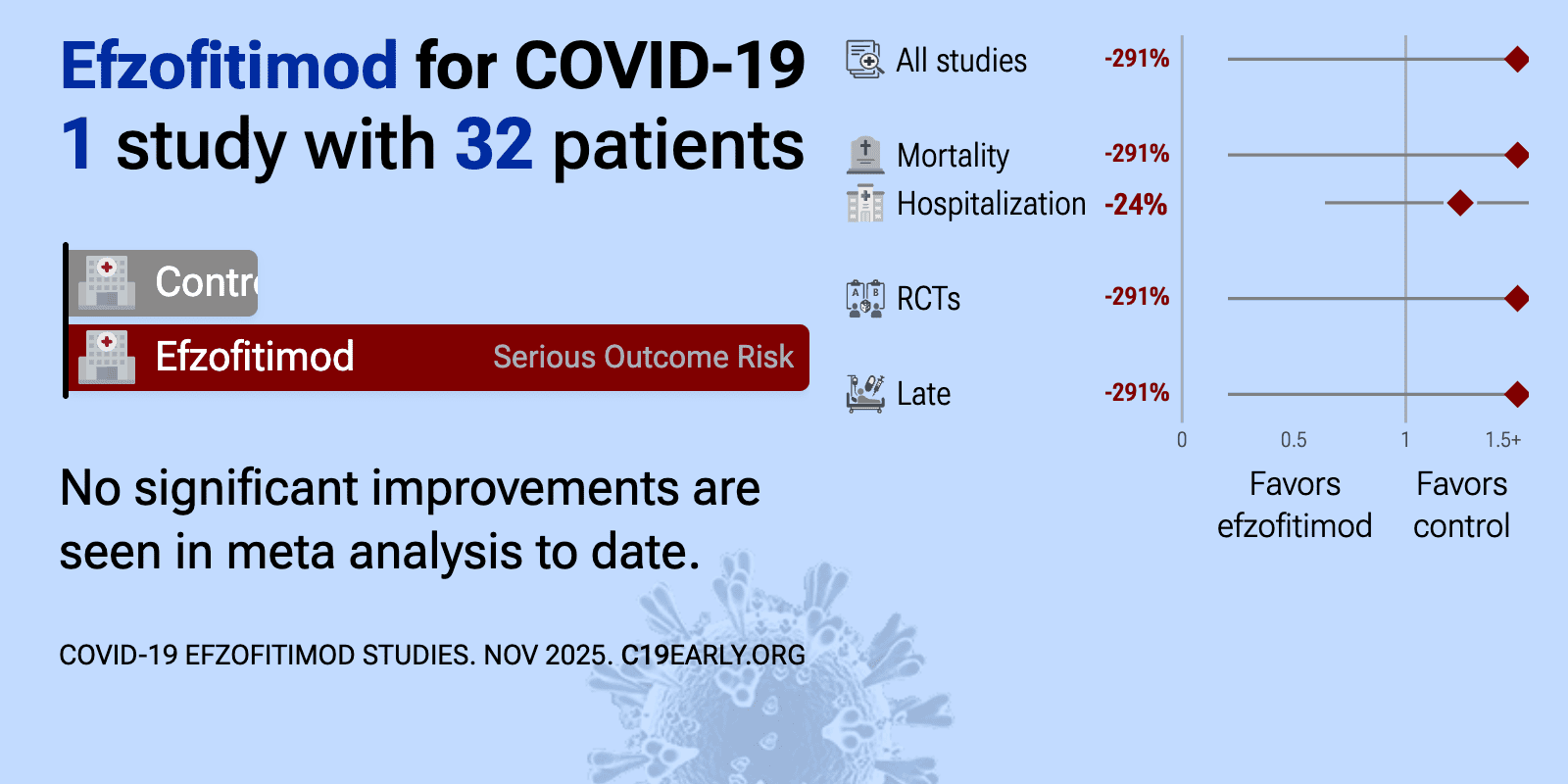
Efzofitimod is an intravenously administered Fc-fusion biologic immunomodulator that binds neuropilin-2 to down-regulate inflammatory responses.
Oct 23 2020 |
et al., NCT04412668 | A Randomized Double-Blind Placebo-Controlled Study to Evaluate the Safety and Efficacy of ATYR1923 In Adult Patients With Severe Pneumonia Related to SARS-CoV-2 Infection (COVID-19) |
| 291% higher mortality (p=1), 24% longer hospitalization (p=0.53), and 11% worse recovery (p=1). RCT 32 hospitalized COVID-19 patients showing no significant differences with efzofitimod. | ||