Impact of androgen deprivation therapy on mortality of prostate cancer patients with COVID-19: a propensity score-based analysis
et al., Infectious Agents and Cancer, doi:10.1186/s13027-021-00406-y, Nov 2021
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
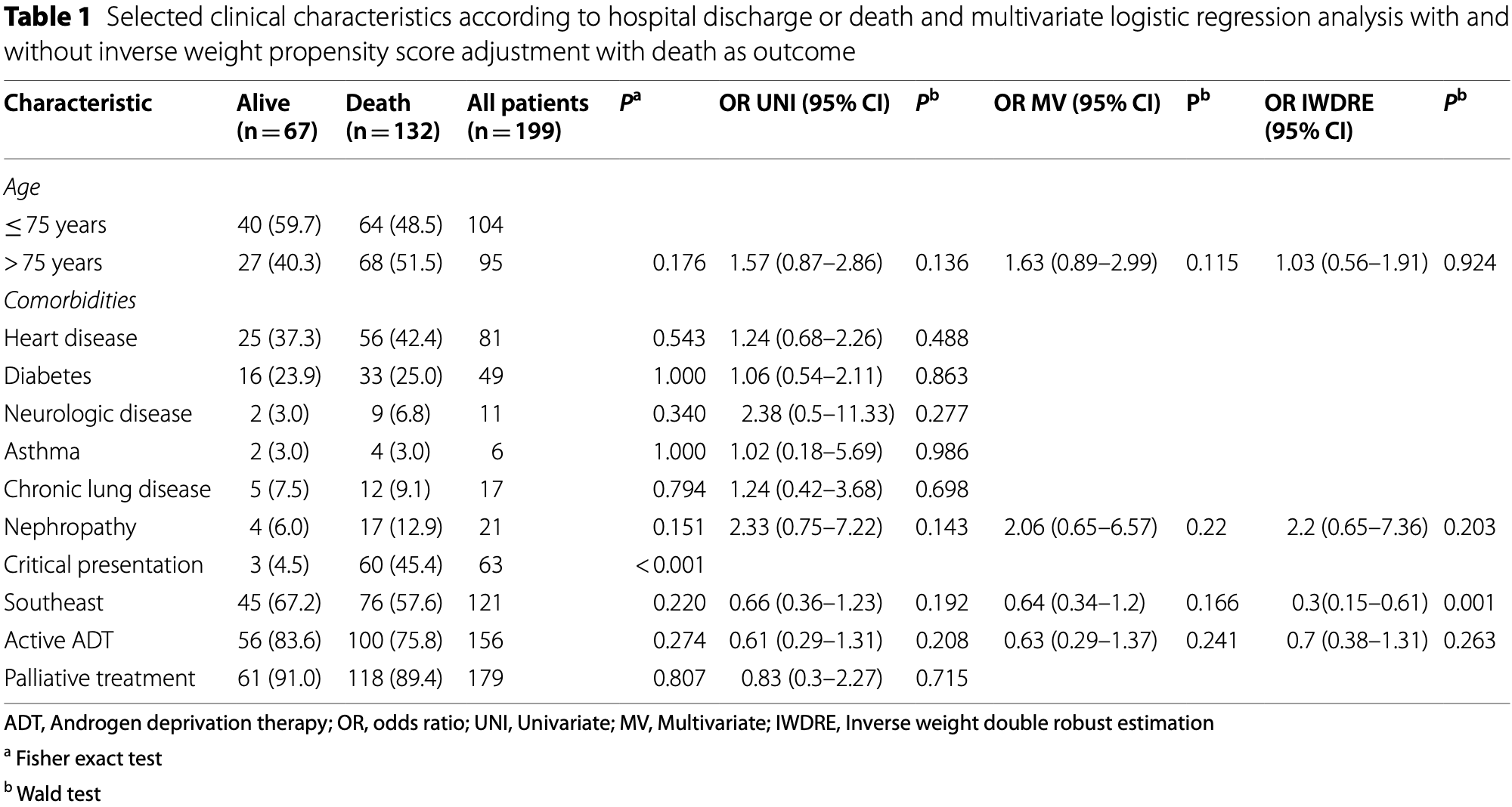
Retrospective 199 prostate cancer patients hospitalized with COVID-19 in Brazil, showing no significant difference in mortality with active ADT.
|
risk of death, 11.2% lower, RR 0.89, p = 0.37, treatment 100 of 156 (64.1%), control 32 of 43 (74.4%), NNT 9.7, adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Duarte et al., 25 Nov 2021, retrospective, Brazil, peer-reviewed, 4 authors.
Impact of androgen deprivation therapy on mortality of prostate cancer patients with COVID-19: a propensity score-based analysis
Infectious Agents and Cancer, doi:10.1186/s13027-021-00406-y
Background: Previous studies hypothesized that androgen deprivation therapy (ADT) may reduce severe acute respiratory syndrome coronavirus 2 (SARS-COV2) infectivity. However, it is unknown whether there is an association between ADT and a higher survival in prostate cancer patients with COVID-19.
Methods: We performed a retrospective analysis of prostate cancer (PC) patients hospitalized to treat COVID-19 in Brazil's public health system. We compared patients with the active use of ADT versus those with non-active ADT, past use. We constructed propensity score models of patients in active versus non-active use of ADT. All variables were used to derive propensity score estimation in both models. In the first model we performed a pair-matched propensity score model between those under active and non-active use of ADT. To the second model we initially performed a multivariate backward elimination process to select variables to a final inverse-weight adjusted with double robust estimation model.
Results: We analyzed 199 PC patients with COVID-19 that received ADT. In total, 52.3% (95/199) of our patients were less than 75 years old, 78.4% (156/199) were on active ADT, and most were using a GnRH analog (80.1%; 125/156). Most of patients were in palliative treatment (89.9%; 179/199). Also, 63.3% of our cohort died from COVID-19. Fortyeight patients under active ADT were pair matched against 48 controls (non-active ADT). All patients (199) were analyzed in the double robust model. ADT active use were not protective factor in both inverse-weight based propensity score (OR 0.70, 95% CI 0.38-1.31, P = 0.263), and pair-matched propensity score (OR 0.67, 95% CI 0.27-1.63, P = 0.374) models. We noticed a significant imbalance in the propensity score of patients in active and those in non-active ADT, with important reductions in the differences after the adjustments.
Conclusions: The active use of ADT was not associated with a reduced risk of death in patients with COVID-19.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s13027-021-00406-y. Additional file 1. Supplemental Table 1 . Baseline variables of active and non-active androgen deprivation therapy groups and standardized mean differences after propensity score-based pair matching. S1 . Standardized mean difference. The mean difference represents the difference between propensity score inside the variable before and after pair matching.
Additional file 2. Figure Additional file 3. Figure S2 . Cumulative distribution of logit propensity score. The graphs summarize the cumulative distribution of logit propensity score, as well as the difference between active and non-active groups before and after matching. Additional file 4. Figure S3 . Density of propensity score distribution. The figure summarizes the distribution of propensity score applied in the double robust estimation model according to the use of androgen deprivation therapy (ADT).
Authors' contributions
Declarations Ethics approval and consent to participate The project was submitted and approved by our institutional ethics (Comitê de Ética em Pesquisa (CEP) da Universidade Estadual de Campinas) commitment and the consent form was waived.
Consent for publication The project was submitted and approved by our institutional ethics (Comitê de Ética em Pesquisa (CEP) da Universidade Estadual de Campinas) commitment and the consent form was waived.
..
References
Angriman, Ferreyro, Burry, Ferguson, Husain, Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context, Lancet Respir Med, doi:10.1016/S2213-2600(21)00139-9
Asselta, Paraboschi, Mantovani, Duga, ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy, Aging
Baden, Sahly, Essink, Kotloff, Frey et al., Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N Engl J Med, doi:10.1056/NEJMoa2035389
Bahmad, Abou-Kheir, Crosstalk between COVID-19 and prostate cancer, Prostate Cancer Prostatic Dis, doi:10.1038/s41391-020-0262-y
Baqui, Bica, Marra, Ercole, Van Der Schaar, Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study, Lancet Glob Heal, doi:10.1016/S2214-109X(20)30285-0
Burki, Equitable distribution of COVID-19 vaccines, Lancet Infect Dis
Caffo, Gasparro, Lorenzo, Volta, Guglielmini et al., Incidence and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with metastatic castration-resistant prostate cancer, Eur J Cancer, doi:10.1016/j.ejca.2020.09.018
Cattrini, Bersanelli, Latocca, Conte, Vallome et al., Sex hormones and hormone therapy during COVID-19 pandemic: implications for patients with cancer, Cancers
Chakravarty, Nair, Hammouda, Ratnani, Gharib et al., Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer, Commun Biol, doi:10.1038/s42003-020-1088-9
Chen, Jeung, Stephenson, Leung, Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor γ-chain messenger ribonucleic acids that are regulated by GnRH in vitro 1, J Clin Endocrinol Metab, doi:10.1210/jcem.84.2.5440
Chen, Jeung, Stephenson, Leung, Ribonucleic acids that are regulated by GnRH in vitro, Endocrinol Metab
Dai, Zhang, Wang, He, Liang et al., Comprehensive analysis of two potential novel SARS-CoV-2 entries, TMPRSS2 and IFITM3, in healthy individuals and cancer patients, Int J Biol Sci
Duarte, Leal, Argenton, Carvalheira, Outcomes of COVID-19 patients under cytotoxic cancer chemotherapy in Brazil, Cancers
Goren, Vaño-Galván, Wambier, Mccoy, Gomez-Zubiaur et al., A preliminary observation: male pattern hair among hospitalized COVID-19 patients in Spain-A potential clue to the role of androgens in COVID-19 severity, J Cosmet Dermatol, doi:10.1111/jocd.13443
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Hou, Zhao, Martin, Kallianpur, Chung et al., New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis, BMC Med, doi:10.1186/s12916-020-01673-z
Karimi, Nowroozi, Alilou, Amini, Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis, Urol J, doi:10.22037/uj.v18i.6691
Khalifa, Mohamed, Elashal, Du, Guo et al., Comprehensive overview on multiple strategies fighting covid-19, Int J Environ Res Public Health
Klein, Li, Milinovich, Schold, Sharifi et al., Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2, J Urol, doi:10.1097/JU.0000000000001338
Ko, Huang, Lin, Juan, Lan et al., Androgeninduced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis, Cancer Res
Kåss, Hollan, Fagerland, Gulseth, Torjesen et al., Rapid anti-inflammatory effects of gonadotropin-releasing hormone antagonism in rheumatoid arthritis patients with high gonadotropin levels in the AGRA trial, PLoS ONE, doi:10.1371/journal.pone.0139439
Lee, Cazier, Starkey, Briggs, Arnold et al., COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study, Lancet Oncol
Litwin, Tan, The diagnosis and treatment of prostate cancer, JAMA
Luque-Fernandez, Belot, Valeri, Cerulli, Maringe et al., Data-adaptive estimation for double-robust methods in populationbased cancer epidemiology: risk differences for lung cancer mortality by emergency presentation, Am J Epidemiol
Min, Park, Lee, Kim, Park, Gonadotropin-releasing hormone modulates immune system function via the nuclear factor-κB pathway in murine Raw264.7 macrophages, NeuroImmunoModulation
Montopoli, Zumerle, Vettor, Rugge, Zorzi et al., Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532), Ann Oncol
Nguyen, Collins, Spence, Devereaux, Daurès et al., Comparison of the ability of double-robust estimators to correct bias in propensity score matching analysis. A Monte Carlo simulation study, Pharmacoepidemiol Drug Saf
Polack, Thomas, Kitchin, Absalon, Gurtman et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Pradhan, Olsson, Sex differences in severity and mortality from COVID-19: are males more vulnerable?, Biol Sex Differ
Qiao, Wang, Mannan, Pitchiaya, Zhang et al., Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2, Proc Natl Acad Sci, doi:10.1073/pnas.2021450118
Ranzani, Bastos, Gelli, Marchesi, Baião et al., Characterisation of the first 250000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data, Lancet Respir Med
Roved, Westerdahl, Hasselquist, Sex differences in immune responses: hormonal effects, antagonistic selection, and evolutionary consequences, Horm Behav, doi:10.1016/j.yhbeh.2016.11.017
Samuel, Majd, Richter, Ghazizadeh, Zekavat et al., Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men, Cell Stem Cell, doi:10.1016/j.stem.2020.11.009
Seeland, Coluzzi, Simmaco, Mura, Bourne et al., Evidence for treatment with estradiol for women with SARS-CoV-2 infection, BMC Med
Sung, García, Dambaeva, Beaman, Gilman-Sachs et al., Gonadotropin-releasing hormone analogues lead to pro-inflammatory changes in T lymphocytes, Am J Reprod Immunol
Tran, Yiannoutsos, Wools-Kaloustian, Siika, Van Der Laan et al., Double robust efficient estimators of longitudinal treatment effects: comparative performance in simulations and a case study, Int J Biostat, doi:10.1515/ijb-2017-0054
Voysey, Clemens, Madhi, Weckx, Folegatti et al., Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Wambier, Goren, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated, J Am Acad Dermatol
Wang, Wang, Zhang, Hu, Zhi et al., Significance of the TMPRSS2: ERG gene fusion in prostate cancer, Mol Med Rep
Wise-Draper, Desai, Elkrief, Rini, Flora et al., LBA71 systemic cancer treatment-related outcomes in patients with SARS-CoV-2 infection: A CCC19 registry analysis, Ann Oncol
Woolf, Chapman, Lee, COVID-19 as the leading cause of death in the United States, JAMA
Zipeto, Da Palmeira, Argañaraz, Argañaraz, ACE2/ADAM17/ TMPRSS2 interplay may be the main risk factor for COVID-19, Front Immunol
Özdemir, Dotto, Sex hormones and anticancer immunity, Clin Cancer Res
DOI record:
{
"DOI": "10.1186/s13027-021-00406-y",
"ISSN": [
"1750-9378"
],
"URL": "http://dx.doi.org/10.1186/s13027-021-00406-y",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Previous studies hypothesized that androgen deprivation therapy (ADT) may reduce severe acute respiratory syndrome coronavirus 2 (SARS-COV2) infectivity. However, it is unknown whether there is an association between ADT and a higher survival in prostate cancer patients with COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a retrospective analysis of prostate cancer (PC) patients hospitalized to treat COVID-19 in Brazil’s public health system. We compared patients with the active use of ADT versus those with non-active ADT, past use. We constructed propensity score models of patients in active versus non-active use of ADT. All variables were used to derive propensity score estimation in both models. In the first model we performed a pair-matched propensity score model between those under active and non-active use of ADT. To the second model we initially performed a multivariate backward elimination process to select variables to a final inverse-weight adjusted with double robust estimation model.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>We analyzed 199 PC patients with COVID-19 that received ADT. In total, 52.3% (95/199) of our patients were less than 75 years old, 78.4% (156/199) were on active ADT, and most were using a GnRH analog (80.1%; 125/156). Most of patients were in palliative treatment (89.9%; 179/199). Also, 63.3% of our cohort died from COVID-19. Forty-eight patients under active ADT were pair matched against 48 controls (non-active ADT). All patients (199) were analyzed in the double robust model. ADT active use were not protective factor in both inverse-weight based propensity score (OR 0.70, 95% CI 0.38–1.31, <jats:italic>P</jats:italic> = 0.263), and pair-matched propensity score (OR 0.67, 95% CI 0.27–1.63, <jats:italic>P</jats:italic> = 0.374) models. We noticed a significant imbalance in the propensity score of patients in active and those in non-active ADT, with important reductions in the differences after the adjustments.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>The active use of ADT was not associated with a reduced risk of death in patients with COVID-19.</jats:p>\n </jats:sec>",
"alternative-id": [
"406"
],
"article-number": "66",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "9 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "11 November 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "25 November 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The project was submitted and approved by our institutional ethics (Comitê de Ética em Pesquisa (CEP) da Universidade Estadual de Campinas) commitment and the consent form was waived."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The project was submitted and approved by our institutional ethics (Comitê de Ética em Pesquisa (CEP) da Universidade Estadual de Campinas) commitment and the consent form was waived."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Duarte",
"given": "Mateus Bringel Oliveira",
"sequence": "first"
},
{
"affiliation": [],
"family": "Leal",
"given": "Frederico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Argenton",
"given": "Juliana Luz Passos",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0136-0943",
"affiliation": [],
"authenticated-orcid": false,
"family": "Carvalheira",
"given": "José Barreto Campello",
"sequence": "additional"
}
],
"container-title": [
"Infectious Agents and Cancer"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
30
]
],
"date-time": "2021-11-30T18:32:03Z",
"timestamp": 1638297123000
},
"deposited": {
"date-parts": [
[
2021,
11,
30
]
],
"date-time": "2021-11-30T18:40:32Z",
"timestamp": 1638297632000
},
"funder": [
{
"DOI": "10.13039/501100001807",
"award": [
"2018/23428‐0"
],
"doi-asserted-by": "publisher",
"name": "fundação de amparo à pesquisa do estado de são paulo"
}
],
"indexed": {
"date-parts": [
[
2021,
12,
10
]
],
"date-time": "2021-12-10T03:28:51Z",
"timestamp": 1639106931736
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1750-9378"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2021,
11,
25
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
25
]
],
"date-time": "2021-11-25T00:00:00Z",
"timestamp": 1637798400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
25
]
],
"date-time": "2021-11-25T00:00:00Z",
"timestamp": 1637798400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13027-021-00406-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13027-021-00406-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13027-021-00406-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2021,
11,
25
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
25
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"author": "SH Woolf",
"first-page": "123",
"journal-title": "JAMA",
"key": "406_CR1",
"unstructured": "Woolf SH, Chapman DA, Lee JH. COVID-19 as the leading cause of death in the United States. JAMA. 2020;325:123–4.",
"volume": "325",
"year": "2020"
},
{
"key": "406_CR2",
"unstructured": "World Health Organization COVID-19 [Internet]. [cited 2021 November 05]. Available from: https://covid19.who.int"
},
{
"DOI": "10.1016/S2213-2600(21)00139-9",
"author": "F Angriman",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "406_CR3",
"unstructured": "Angriman F, Ferreyro BL, Burry L, Fan E, Ferguson ND, Husain S, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir Med. 2021. https://doi.org/10.1016/S2213-2600(21)00139-9.",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"author": "M Voysey",
"doi-asserted-by": "publisher",
"first-page": "99",
"journal-title": "Lancet",
"key": "406_CR4",
"unstructured": "Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99–111.",
"volume": "397",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2034577",
"author": "FP Polack",
"doi-asserted-by": "publisher",
"first-page": "2603",
"issue": "27",
"journal-title": "N Engl J Med",
"key": "406_CR5",
"unstructured": "Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035389",
"author": "LR Baden",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "406_CR6",
"unstructured": "Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2035389.",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30949-X",
"author": "T Burki",
"doi-asserted-by": "publisher",
"first-page": "33",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "406_CR7",
"unstructured": "Burki T. Equitable distribution of COVID-19 vaccines. Lancet Infect Dis. 2021;21(1):33–4.",
"volume": "21",
"year": "2021"
},
{
"author": "SAM Khalifa",
"first-page": "1",
"issue": "16",
"journal-title": "Int J Environ Res Public Health",
"key": "406_CR8",
"unstructured": "Khalifa SAM, Mohamed BS, Elashal MH, Du M, Guo Z, Zhao C, et al. Comprehensive overview on multiple strategies fighting covid-19. Int J Environ Res Public Health. 2020;17(16):1–13.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "W Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "406_CR9",
"unstructured": "Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. https://doi.org/10.1056/NEJMoa2002032.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1186/s12916-020-01851-z",
"author": "U Seeland",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "BMC Med",
"key": "406_CR10",
"unstructured": "Seeland U, Coluzzi F, Simmaco M, Mura C, Bourne PE, Heiland M, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18(1):1–9.",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1111/jocd.13443",
"author": "A Goren",
"doi-asserted-by": "publisher",
"first-page": "1545",
"issue": "7",
"journal-title": "J Cosmet Dermatol [Internet]",
"key": "406_CR11",
"unstructured": "Goren A, Vaño-Galván S, Wambier CG, McCoy J, Gomez-Zubiaur A, Moreno-Arrones OM, et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain—A potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol [Internet]. 2020;19(7):1545–7. https://doi.org/10.1111/jocd.13443.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.04.032",
"author": "CG Wambier",
"doi-asserted-by": "publisher",
"first-page": "308",
"issue": "1",
"journal-title": "J Am Acad Dermatol",
"key": "406_CR12",
"unstructured": "Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83(1):308–9.",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2021450118",
"author": "Y Qiao",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Proc Natl Acad Sci",
"key": "406_CR13",
"unstructured": "Qiao Y, Wang X, Mannan R, Pitchiaya S, Zhang Y, Wotring JW, et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci. 2021;118(1): e2021450118. https://doi.org/10.1073/pnas.2021450118.",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1016/j.stem.2020.11.009",
"author": "RM Samuel",
"doi-asserted-by": "publisher",
"first-page": "876",
"issue": "6",
"journal-title": "Cell Stem Cell",
"key": "406_CR14",
"unstructured": "Samuel RM, Majd H, Richter MN, Ghazizadeh Z, Zekavat SM, Navickas A, et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27(6):876-889.e12. https://doi.org/10.1016/j.stem.2020.11.009.",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.3390/cancers12082325",
"author": "C Cattrini",
"doi-asserted-by": "publisher",
"first-page": "2325",
"issue": "8",
"journal-title": "Cancers (Basel)",
"key": "406_CR15",
"unstructured": "Cattrini C, Bersanelli M, Latocca MM, Conte B, Vallome G, Boccardo F. Sex hormones and hormone therapy during COVID-19 pandemic: implications for patients with cancer. Cancers (Basel). 2020;12(8):2325.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.7150/ijbs.51234",
"author": "Y-J Dai",
"doi-asserted-by": "publisher",
"first-page": "3028",
"issue": "15",
"journal-title": "Int J Biol Sci",
"key": "406_CR16",
"unstructured": "Dai Y-J, Zhang W-N, Wang W-D, He S-Y, Liang C-C, Wang D-W. Comprehensive analysis of two potential novel SARS-CoV-2 entries, TMPRSS2 and IFITM3, in healthy individuals and cancer patients. Int J Biol Sci. 2020;16(15):3028–36.",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1001/jama.2017.7248",
"author": "MS Litwin",
"doi-asserted-by": "publisher",
"first-page": "2532",
"issue": "24",
"journal-title": "JAMA",
"key": "406_CR17",
"unstructured": "Litwin MS, Tan H-J. The diagnosis and treatment of prostate cancer. JAMA. 2017;317(24):2532.",
"volume": "317",
"year": "2017"
},
{
"DOI": "10.1186/s13293-020-00330-7",
"author": "A Pradhan",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Biol Sex Differ",
"key": "406_CR18",
"unstructured": "Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ. 2020;11(1):1–11.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41391-020-0262-y",
"author": "HF Bahmad",
"doi-asserted-by": "publisher",
"first-page": "561",
"issue": "4",
"journal-title": "Prostate Cancer Prostatic Dis",
"key": "406_CR19",
"unstructured": "Bahmad HF, Abou-Kheir W. Crosstalk between COVID-19 and prostate cancer. Prostate Cancer Prostatic Dis. 2020;23(4):561–3. https://doi.org/10.1038/s41391-020-0262-y.",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1038/s42003-020-1088-9",
"author": "D Chakravarty",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Commun Biol",
"key": "406_CR20",
"unstructured": "Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol. 2020;3(1):1–12. https://doi.org/10.1038/s42003-020-1088-9.",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"author": "M Montopoli",
"doi-asserted-by": "publisher",
"first-page": "1040",
"issue": "8",
"journal-title": "Ann Oncol",
"key": "406_CR21",
"unstructured": "Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040–5.",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1097/JU.0000000000001338",
"author": "EA Klein",
"doi-asserted-by": "publisher",
"journal-title": "J Urol",
"key": "406_CR22",
"unstructured": "Klein EA, Li J, Milinovich A, Schold JD, Sharifi N, Kattan MW, et al. Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2. J Urol. 2020. https://doi.org/10.1097/JU.0000000000001338.",
"year": "2020"
},
{
"DOI": "10.3390/cancers12123490",
"author": "MBO Duarte",
"doi-asserted-by": "publisher",
"first-page": "3490",
"issue": "12",
"journal-title": "Cancers (Basel)",
"key": "406_CR23",
"unstructured": "Duarte MBO, Leal F, Argenton JLP, Carvalheira JBC. Outcomes of COVID-19 patients under cytotoxic cancer chemotherapy in Brazil. Cancers (Basel). 2020;12(12):3490.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1515/ijb-2017-0054",
"author": "L Tran",
"doi-asserted-by": "publisher",
"journal-title": "Int J Biostat",
"key": "406_CR24",
"unstructured": "Tran L, Yiannoutsos C, Wools-Kaloustian K, Siika A, Van Der Laan M, Petersen M. Double robust efficient estimators of longitudinal treatment effects: comparative performance in simulations and a case study. Int J Biostat. 2019. https://doi.org/10.1515/ijb-2017-0054.",
"year": "2019"
},
{
"DOI": "10.1002/pds.4325",
"author": "TL Nguyen",
"doi-asserted-by": "publisher",
"first-page": "1513",
"issue": "12",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "406_CR25",
"unstructured": "Nguyen TL, Collins GS, Spence J, Devereaux PJ, Daurès JP, Landais P, et al. Comparison of the ability of double-robust estimators to correct bias in propensity score matching analysis. A Monte Carlo simulation study. Pharmacoepidemiol Drug Saf. 2017;26(12):1513–9.",
"volume": "26",
"year": "2017"
},
{
"DOI": "10.1093/aje/kwx317",
"author": "MA Luque-Fernandez",
"doi-asserted-by": "publisher",
"first-page": "871",
"issue": "4",
"journal-title": "Am J Epidemiol",
"key": "406_CR26",
"unstructured": "Luque-Fernandez MA, Belot A, Valeri L, Cerulli G, Maringe C, Rachet B. Data-adaptive estimation for double-robust methods in population-based cancer epidemiology: risk differences for lung cancer mortality by emergency presentation. Am J Epidemiol. 2018;187(4):871–8.",
"volume": "187",
"year": "2018"
},
{
"DOI": "10.18632/aging.103415",
"author": "R Asselta",
"doi-asserted-by": "publisher",
"first-page": "10087",
"issue": "11",
"journal-title": "Aging (Albany NY)",
"key": "406_CR27",
"unstructured": "Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY). 2020;12(11):10087–98.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1186/s12916-020-01673-z",
"author": "Y Hou",
"doi-asserted-by": "publisher",
"first-page": "216",
"issue": "1",
"journal-title": "BMC Med",
"key": "406_CR28",
"unstructured": "Hou Y, Zhao J, Martin W, Kallianpur A, Chung MK, Jehi L, et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18(1):216. https://doi.org/10.1186/s12916-020-01673-z.",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.576745",
"author": "D Zipeto",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Front Immunol",
"key": "406_CR29",
"unstructured": "Zipeto D, da Palmeira JF, Argañaraz GA, Argañaraz ER. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front Immunol. 2020;11:1–10.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3892/mmr.2017.7281",
"author": "Z Wang",
"doi-asserted-by": "publisher",
"first-page": "5450",
"issue": "4",
"journal-title": "Mol Med Rep",
"key": "406_CR30",
"unstructured": "Wang Z, Wang Y, Zhang J, Hu Q, Zhi F, Zhang S, et al. Significance of the TMPRSS2: ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16(4):5450–8.",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1158/0008-5472.CAN-14-3297",
"author": "CJ Ko",
"doi-asserted-by": "publisher",
"first-page": "2949",
"issue": "14",
"journal-title": "Cancer Res",
"key": "406_CR31",
"unstructured": "Ko CJ, Huang CC, Lin HY, Juan CP, Lan SW, Shyu HY, et al. Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res. 2015;75(14):2949–60.",
"volume": "75",
"year": "2015"
},
{
"DOI": "10.1016/j.ejca.2020.09.018",
"author": "O Caffo",
"doi-asserted-by": "publisher",
"first-page": "140",
"journal-title": "Eur J Cancer",
"key": "406_CR32",
"unstructured": "Caffo O, Gasparro D, Di Lorenzo G, Volta AD, Guglielmini P, Zucali P, et al. Incidence and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with metastatic castration-resistant prostate cancer. Eur J Cancer. 2020;140:140–6. https://doi.org/10.1016/j.ejca.2020.09.018.",
"volume": "140",
"year": "2020"
},
{
"DOI": "10.1016/j.yhbeh.2016.11.017",
"author": "J Roved",
"doi-asserted-by": "publisher",
"first-page": "95",
"journal-title": "Horm Behav",
"key": "406_CR33",
"unstructured": "Roved J, Westerdahl H, Hasselquist D. Sex differences in immune responses: hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav. 2017;88:95–105. https://doi.org/10.1016/j.yhbeh.2016.11.017.",
"volume": "88",
"year": "2017"
},
{
"DOI": "10.1158/1078-0432.CCR-19-0137",
"author": "BC Özdemir",
"doi-asserted-by": "publisher",
"first-page": "4603",
"issue": "15",
"journal-title": "Clin Cancer Res",
"key": "406_CR34",
"unstructured": "Özdemir BC, Dotto GP. Sex hormones and anticancer immunity. Clin Cancer Res. 2019;25(15):4603–10.",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1210/jcem.84.2.5440",
"author": "H-F Chen",
"doi-asserted-by": "publisher",
"first-page": "743",
"issue": "2",
"journal-title": "J Clin Endocrinol Metab",
"key": "406_CR35",
"unstructured": "Chen H-F, Jeung E-B, Stephenson M, Leung PCK. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor γ-chain messenger ribonucleic acids that are regulated by GnRH in vitro 1. J Clin Endocrinol Metab. 1999;84(2):743–50. https://doi.org/10.1210/jcem.84.2.5440.",
"volume": "84",
"year": "1999"
},
{
"author": "H Chen",
"first-page": "743",
"issue": "2",
"journal-title": "Endocrinol Metab",
"key": "406_CR36",
"unstructured": "Chen H, Jeung E, Stephenson M, Leung PCK. Ribonucleic acids that are regulated by GnRH in vitro. Endocrinol Metab. 1999;84(2):743–50.",
"volume": "84",
"year": "1999"
},
{
"DOI": "10.1159/000204231",
"author": "JY Min",
"doi-asserted-by": "publisher",
"first-page": "177",
"issue": "3",
"journal-title": "NeuroImmunoModulation",
"key": "406_CR37",
"unstructured": "Min JY, Park MH, Lee JK, Kim HJ, Park YK. Gonadotropin-releasing hormone modulates immune system function via the nuclear factor-κB pathway in murine Raw264.7 macrophages. NeuroImmunoModulation. 2009;16(3):177–84.",
"volume": "16",
"year": "2009"
},
{
"DOI": "10.1111/aji.12522",
"author": "N Sung",
"doi-asserted-by": "publisher",
"first-page": "50",
"issue": "1",
"journal-title": "Am J Reprod Immunol",
"key": "406_CR38",
"unstructured": "Sung N, Salazar García MD, Dambaeva S, Beaman KD, Gilman-Sachs A, Kwak-Kim J. Gonadotropin-releasing hormone analogues lead to pro-inflammatory changes in T lymphocytes. Am J Reprod Immunol. 2016;76(1):50–8.",
"volume": "76",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0139439",
"author": "A Kåss",
"doi-asserted-by": "publisher",
"first-page": "e0139439",
"issue": "10",
"journal-title": "PLoS ONE",
"key": "406_CR39",
"unstructured": "Kåss A, Hollan I, Fagerland MW, Gulseth HC, Torjesen PA, Førre ØT. Rapid anti-inflammatory effects of gonadotropin-releasing hormone antagonism in rheumatoid arthritis patients with high gonadotropin levels in the AGRA trial. PLoS ONE. 2015;10(10):e0139439. https://doi.org/10.1371/journal.pone.0139439.",
"volume": "10",
"year": "2015"
},
{
"author": "LYW Lee",
"first-page": "1",
"issue": "20",
"journal-title": "Lancet Oncol",
"key": "406_CR40",
"unstructured": "Lee LYW, Cazier J-B, Starkey T, Briggs SEW, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;2045(20):1–8.",
"volume": "2045",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.08.2312",
"author": "TM Wise-Draper",
"doi-asserted-by": "publisher",
"first-page": "S1201",
"journal-title": "Ann Oncol",
"key": "406_CR41",
"unstructured": "Wise-Draper TM, Desai A, Elkrief A, Rini BI, Flora DB, Bowles DW, et al. LBA71 systemic cancer treatment-related outcomes in patients with SARS-CoV-2 infection: A CCC19 registry analysis. Ann Oncol. 2020;31:S1201–2.",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.22037/uj.v18i.6691",
"author": "A Karimi",
"doi-asserted-by": "publisher",
"journal-title": "Urol J",
"key": "406_CR42",
"unstructured": "Karimi A, Nowroozi A, Alilou S, Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol J. 2021. https://doi.org/10.22037/uj.v18i.6691.",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(20)30285-0",
"author": "P Baqui",
"doi-asserted-by": "publisher",
"first-page": "e1018",
"issue": "8",
"journal-title": "Lancet Glob Heal",
"key": "406_CR43",
"unstructured": "Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Heal. 2020;8(8):e1018–26. https://doi.org/10.1016/S2214-109X(20)30285-0.",
"volume": "8",
"year": "2020"
},
{
"author": "OT Ranzani",
"first-page": "1",
"issue": "20",
"journal-title": "Lancet Respir Med",
"key": "406_CR44",
"unstructured": "Ranzani OT, Bastos LSL, Gelli JGM, Marchesi JF, Baião F, Hamacher S, et al. Characterisation of the first 250000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;2600(20):1–12.",
"volume": "2600",
"year": "2021"
},
{
"DOI": "10.1177/2042018820969019",
"author": "S Salciccia",
"doi-asserted-by": "publisher",
"first-page": "204201882096901",
"journal-title": "Ther Adv Endocrinol Metab",
"key": "406_CR45",
"unstructured": "Salciccia S, Del Giudice F, Eisenberg ML, Mastroianni CM, De Berardinis E, Ricciuti GP, et al. Androgen-deprivation therapy and SARS-Cov-2 infection: the potential double-face role of testosterone. Ther Adv Endocrinol Metab. 2020;11:204201882096901. https://doi.org/10.1177/2042018820969019.",
"volume": "11",
"year": "2020"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"score": 1,
"short-container-title": [
"Infect Agents Cancer"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Cancer Research",
"Infectious Diseases",
"Oncology",
"Epidemiology"
],
"subtitle": [],
"title": [
"Impact of androgen deprivation therapy on mortality of prostate cancer patients with COVID-19: a propensity score-based analysis"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "16"
}
