Primary cilium and TULP3-dependent ciliary targeting of ACE2 in SARS-CoV-2 tropism
et al., Cell Communication and Signaling, doi:10.1186/s12964-025-02519-y, Nov 2025
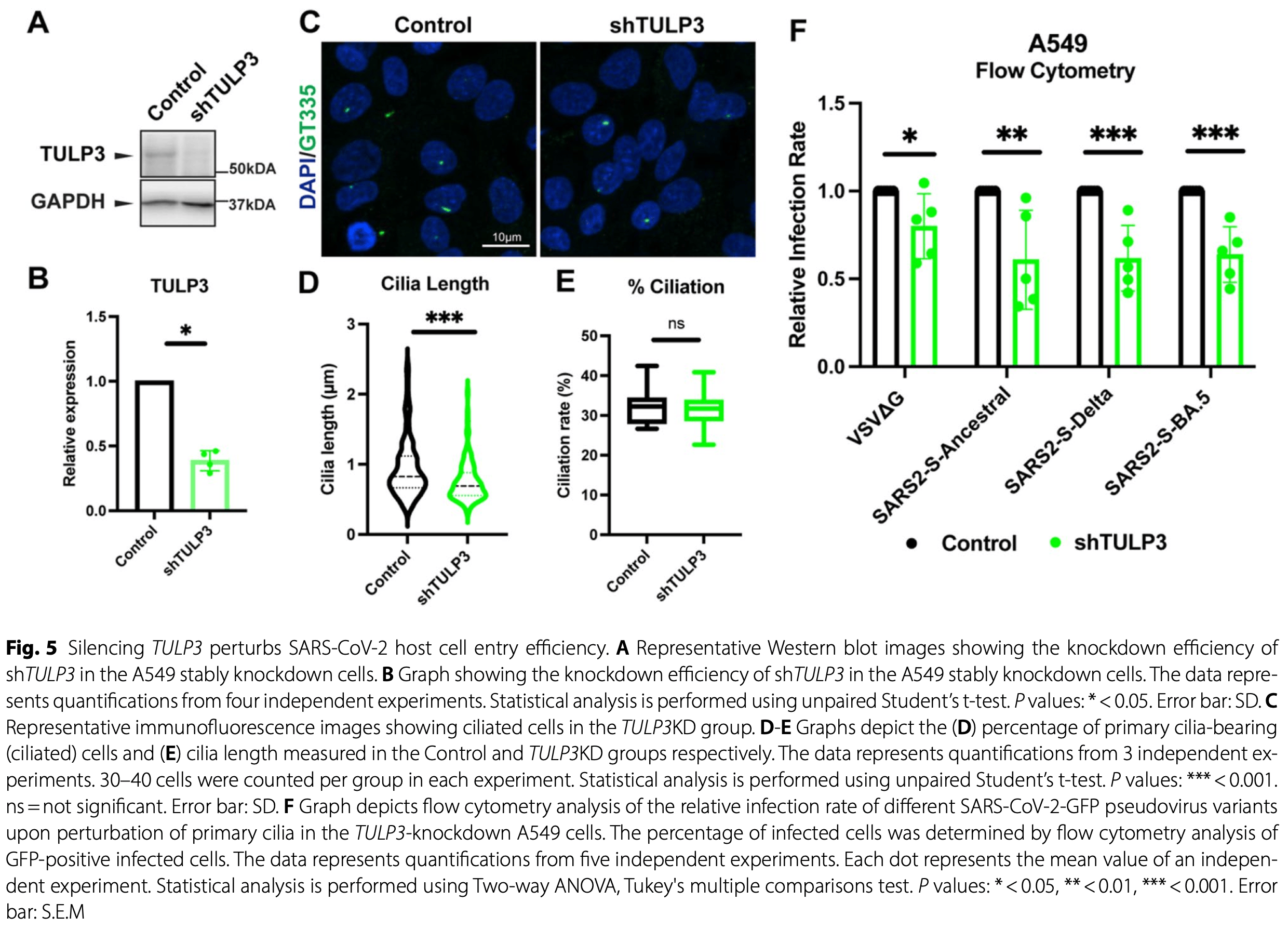
In vitro study showing that TULP3-mediated ACE2 ciliary targeting facilitates SARS-CoV-2 infection in human lung and retinal epithelial cells. Authors find that TULP3 physically interacts with ACE2 and directs its localization to primary cilia, where viral spike proteins preferentially accumulate. TULP3 knockdown reduces ACE2 ciliary enrichment and significantly decreases infection rates of ancestral, Delta, and Omicron BA.5 variants in A549 lung cells and hTERT-RPE1 retinal cells by 20-40%. Disruption of primary cilia formation through ARL13B or IFT88 knockdown similarly reduces viral entry efficiency. Authors demonstrate that ACE2 and NRP1 are enriched on primary cilia across multiple SARS-CoV-2 susceptible cell types including lung epithelial (A549), retinal pigmented epithelial (hTERT-RPE1), neuroblastoma (SH-SY5Y), and human iPSC-derived neural progenitor cells. Analysis of COVID-19 patient lung samples reveals dysregulation of 129 ciliary genes including IFT complex components and ciliary trafficking machinery. Co-immunoprecipitation confirms direct TULP3-ACE2 interaction, while AlphaFold3 modeling predicts specific binding interfaces. The study identifies primary cilia as concentration hubs for viral entry factors and suggests that TULP3-mediated ACE2 trafficking represents a novel therapeutic target for reducing SARS-CoV-2 tropism without affecting systemic ACE2 function.
Du et al., 28 Nov 2025, China, peer-reviewed, 13 authors.
Contact: catherinehor@hkbu.edu.hk.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Primary cilium and TULP3-dependent ciliary targeting of ACE2 in SARS-CoV-2 tropism
Cell Communication and Signaling, doi:10.1186/s12964-025-02519-y
Background Viruses initiate infection by engaging specific receptors on the host cell surface. While the surface receptor ACE2 mediates SARS-CoV-2 entry, the precise role of subcellular trafficking, particularly, the exact involvement of primary cilium trafficking in viral entry remains largely unresolved.
Methods We used in-vitro human cell models and SARS-CoV-2 pseudoviruses to elucidate the viral attachment and host cell entry mechanisms. Mechanistic studies were conducted using a multidimensional approach that combined flow cytometry analysis, co-immunofluorescence and confocal microscopy, co-immunoprecipitation, genetic manipulations and ciliary perturbations, and structural predictions.
Results Our study uncovers Tubby Like Protein-3 (TULP3) as a pivotal ciliary trafficking adaptor that facilitates ACE2 localization to the primary cilium. We show that ACE2 and TULP3 physically associate, and that TULP3 depletion not only removes ACE2 from the ciliary axoneme but also impairs SARS-CoV-2 pseudovirus entry. This ACE2 localization is partially dependent on TULP3's interaction with the IFT-A complex, as an IFT-A-binding-deficient TULP3 mutant could still partially rescue ciliary ACE2 levels. Furthermore, genetic disruption of ACE2-enriched primary cilia in human lung cells and retinal pigment epithelial cells significantly diminishes the infectivity of SARS-CoV-2 pseudoviruses, including the ancestral, Delta and Omicron BA.5 variants. Viral spike protein attachment assays reveal preferential binding and accumulation of the SARS-CoV-2 spike on ACE2-rich ciliary axonemes. Moreover, we demonstrate variable endogenous enrichment of ACE2 within the primary cilium axoneme across diverse SARS-CoV-2 susceptible human cell types, including lung epithelial cells, retinal pigmented epithelial cells, neuroblastoma cells, and human iPSC-derived neural progenitors, offering a potential mechanistic framework for tissue-specific susceptibility and the heterogeneous clinical manifestations of COVID-19.
Conclusion Our findings demonstrate the first evidence of a dedicated ciliary trafficking machinery for ACE2. We provide compelling evidence that SARS-CoV-2 hijacks evolutionarily conserved ciliary trafficking pathways, with TULP3-dependent targeting of ACE2 to primary cilia serving as a determinant of viral host cell tropism and invasion.
Abbreviations
Declarations Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abramson, Adler, Dunger, Evans, Green et al., Accurate structure prediction of biomolecular interactions with AlphaFold 3, Nature, doi:10.1038/s41586-024-07487-w
Ahn, Kim, Hong, Choi, Yang et al., Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19, J Clin Invest, doi:10.1172/JCI148517
Badgandi, Hwang, Shimada, Loriot, Mukhopadhyay, Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins, J Cell Biol, doi:10.1083/jcb.201607095
Bayati, Kumar, Francis, Mcpherson, SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis, J Biol Chem, doi:10.1016/j.jbc.2021.100306
Belgacemi, Diabasana, Hoarau, Dubernard, Mérol et al., Primary ciliogenesis is a crucial step for multiciliated cell determinism in the respiratory epithelium, J Cell Mol Med, doi:10.1111/jcmm.16729
Buqaileh, Saternos, Ley, Aranda, Forero et al., Can cilia provide an entry gateway for SARS-CoV-2 to human ciliated cells?, Physiol Genomics, doi:10.1152/physiolgenomics.00015.2021
Butowt, Bilinska, Bartheld, Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms, Trends Neurosci, doi:10.1016/j.tins.2022.11.003
Cantuti-Castelvetri, Ojha, Pedro, Djannatian, Franz et al., Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity, Science, doi:10.1126/science.abd2985
Daniloski, Jordan, Wessels, Hoagland, Kasela et al., Identification of required host factors for SARS-CoV-2 infection in human cells, Cell, doi:10.1016/j.cell.2020.10.030
E U R O S C I, None, J N, doi:10.1523/JNEUROSCI.3005-20.2021
Fagan, Lee, Geron, Scherrer, Zastrow et al., Selective targeting of Mu opioid receptors to primary cilia, Cell Rep, doi:10.1016/j.celrep.2024.114164
Gonzalez-Rubio, Vtk, Baumann, Cheremkhina, Kubiza et al., SARS-CoV-2 particles promote airway epithelial differentiation and ciliation, Front Bioeng Biotechnol, doi:10.3389/fbioe.2023.1268782
Han, Ko, Shikada, Amano, Wang et al., TULP3 is required for localization of membrane-associated proteins ARL13B and INPP5E to primary cilia, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2018.12.109
Hesketh, Mukhopadhyay, Nakamura, Toropova, Roberts, IFT-a structure reveals carriages for membrane protein transport into cilia, Cell, doi:10.1016/j.cell.2022.11.010
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hong, Kim, Park, Seo, Moon, Differential roles of Tubby family proteins in ciliary formation and trafficking, Mol Cells, doi:10.14348/molcells.2021.0082
Hor, Lo, Cham, Leong, Goh, Multifaceted functions of Rab23 on primary cilium-mediated and Hedgehog signaling-mediated cerebellar granule cell proliferation, J Neurosci, doi:10.1523/JNEUROSCI.3005-20.2021
Ishikawa, Marshall, Intraflagellar transport and ciliary dynamics, Cold Spring Harb Perspect Biol, doi:10.1101/cshperspect.a021998
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol, doi:10.1038/s41580-021-00418-x
Jain, Pan, Driscoll, Wisner, Huang et al., Temporal relationship between primary and motile ciliogenesis in airway epithelial cells, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2009-0328OC
James, Boris, Katja, Kai-En, Williamson et al., Neuropilin-1 is a host factor for SARS-CoV-2 infection, Science, doi:10.1126/science.abd3072
Lalioti, González-Sanz, Bermejo, González-Jiménez, Viedma-Poyatos et al., Cell surface detection of vimentin, ACE2 and SARS-CoV-2 spike proteins reveals selective colocalization at primary cilia, Sci Rep, doi:10.1038/s41598-022-11248-y
Lee, Nakayama, Wu, Goltsev, Jiang et al., ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs, Nat Commun, doi:10.1038/s41467-020-19145-6
Leong, Tung, Wilkie, Hor, Dutcher, RAB23 lossof-function mutation causes context-dependent ciliopathy in Carpenter syndrome, PLoS Genet, doi:10.1371/journal.pgen.1011611
Li, Li, Ou, COVID-19, Cilia, and Smell, FEBS J, doi:10.1111/febs.15491
Liu, Huuskonen, Laitinen, Redchuk, Bogacheva et al., SARS-CoV-2-host proteome interactions for antiviral drug discovery, Mol Syst Biol, doi:10.15252/msb.202110396
Lubbe, Cozier, Oosthuizen, Acharya, Sturrock, ACE2 and ACE: Structure-Based Insights into Mechanism, Regulation and Receptor Recognition by SARS-CoV, Clin Sci, doi:10.1042/CS20200899
Luczka-Majérus, Bonnomet, Germain, Lalun, Kileztky et al., Ciliogenesis is intrinsically altered in COPD small airways, Eur Respir J, doi:10.1183/13993003.00791-2022
Mukhopadhyay, Badgandi, Hwang, Somatilaka, Shimada et al., Trafficking to the primary cilium membrane, Mol Biol Cell, doi:10.1091/mbc.E16-07-0505
Mukhopadhyay, Jackson, The tubby family proteins, Genome Biol, doi:10.1186/gb-2011-12-6-225
Mukhopadhyay, Wen, Chih, Nelson, Lane et al., TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia, Genes Dev, doi:10.1101/GAD.1966210
Oke, Olubunmi O Oladunjoye, Adeolu O Oladunjoye, Paudel, Zimmerman, Bell's palsy as a late neurologic manifestation of COVID-19 infection, Cureus, doi:10.7759/CUREUS.13881
Oughtred, Rust, Chang, Breitkreutz, Stark et al., The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions, Protein Sci, doi:10.1002/pro.3978
Pal, De, Yates, Kolape, Muchero, Mutating novel interaction sites in NRP1 reduces SARS-CoV-2 spike protein internalization, d o i . o r g / 1 0, doi:10.1016/j.isci.2023.106274
Palicharla, Badgandi, Hwang, Legué, Liem et al., A defined tubby domain β-barrel surface region of TULP3 mediates ciliary trafficking of diverse cargoes, Mol Biol Cell, doi:10.1091/mbc.E24-09-0426
Palicharla, Hwang, Somatilaka, Legué, Shimada et al., Interactions between TULP3 tubby domain and ARL13B amphipathic helix promote lipidated protein transport to cilia, Mol Biol Cell, doi:10.1091/mbc.E22-10-0473
Perotin, Coraux, Lagonotte, Birembaut, Delepine et al., Alteration of primary cilia in COPD, Eur Respir J, doi:10.1183/13993003.00122-2018
Pinskey, Franks, Mcmellen, Giger, Allen, Neuropilin-1 promotes Hedgehog signaling through a novel cytoplasmic motif, J Biol Chem, doi:10.1074/jbc.M117.783845
Reiter, Leroux, Genes and molecular pathways underpinning ciliopathies, Nat Rev Mol Cell Biol, doi:10.1038/nrm.2017.60
Robinot, Hubert, De Melo, Lazarini, Bruel et al., SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance, Nat Commun, doi:10.1038/s41467-021-24521-x
S C I T R A N S L M E D, None, A D I, doi:10.1126/SCITRANSLMED.ADI2623
Savelieff, Feldman, Stino, Neurological sequela and disruption of neuron-glia homeostasis in SARS-CoV-2 infection, Neurobiol Dis, doi:10.1016/J.NBD.2022.105715
Schreiner, Allnoch, Beythien, Marek, Becker et al., SARS-CoV-2 infection dysregulates cilia and basal cell homeostasis in the respiratory epithelium of hamsters, Int J Mol Sci, doi:10.3390/ijms23095124
Si, Yao, Ma, Sun, A single-cell transcriptomic landscape of the lungs of patients with COVID-19, Nat Cell Biol, doi:10.1038/s41556-021-00796-6
Singla, Reiter, The primary cilium as the cell's antenna: signaling at a sensory organelle, Science, doi:10.1126/science.1124534
Su, Zhou, Zhang, Motile cilia and microvillar: accomplices of SARS-CoV-2 in penetrating mucus barrier and infecting airway epithelium, Signal Transduct Target Ther, doi:10.1038/s41392-023-01387-7
Taquet, Sillett, Zhu, Mendel, Camplisson et al., Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients, Lancet Psychiatry, doi:10.1016/s2215-0366(22)00260-7
Tilley, Walters, Shaykhiev, Crystal, Cilia dysfunction in lung disease, Annu Rev Physiol, doi:10.1146/annurev-physiol-021014-071931
Torchia, Tavares, Carstensen, Chen, Huang et al., Optimized ACE2 decoys neutralize antibody-resistant SARS-CoV-2 variants through functional receptor mimicry and treat infection in vivo, Sci Adv, doi:10.1126/sciadv.abq6527
Urano, Itoh, Suzuki, Sasaki, Kishikawa et al., An Inhaled ACE2 Decoy Confers Protection against SARS-CoV-2 Infection in Preclinical Models, Science Translational Medicine, doi:10.1126/SCITRANSLMED.ADI2623
Van Dam, Kennedy, Van Der Lee, De Vrieze, Wunderlich et al., Ciliacarta: an integrated and validated compendium of ciliary genes, PLoS One, doi:10.1371/journal.pone.0216705
Van Dam, Wheway, Slaats, Huynen, Giles, The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium, h t t p s : / / d o i . o r g, doi:10.1186/2046-2530-2-7
Wu, Lidsky, Xiao, Cheng, Lee et al., SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming, Cell, doi:10.1016/j.cell.2022.11.030
Zhang, Li, Feng, Ramos, Da Silva et al., SARS-CoV-2 Pseudovirus Infectivity and Expression of Viral Entry-Related Factors ACE2, TMPRSS2, Kim-1, and NRP-1 in Human Cells from the Respiratory, Urinary, Digestive, Reproductive, and Immune Systems, J Med Virol, doi:10.1002/JMV.27244
Zhuang, Cheng, Zhang, Jiang, Li et al., Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-NCoV (or SARS-CoV-2) infection, J Med Virol, doi:10.1002/jmv.26139
DOI record:
{
"DOI": "10.1186/s12964-025-02519-y",
"ISSN": [
"1478-811X"
],
"URL": "http://dx.doi.org/10.1186/s12964-025-02519-y",
"alternative-id": [
"2519"
],
"article-number": "515",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "19 August 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "30 October 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "28 November 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Du",
"given": "Yun Hong",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tung",
"given": "Wai Lam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Ho Hoi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Xuewen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Alexis Shiying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Run",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Zecheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Qian-Yuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Bor Luen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Xing-Lou",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Alwis",
"given": "Ruklanthi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tan",
"given": "Chee Wah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hor",
"given": "Catherine Hong Huan",
"sequence": "additional"
}
],
"container-title": "Cell Communication and Signaling",
"container-title-short": "Cell Commun Signal",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
11,
28
]
],
"date-time": "2025-11-28T12:24:52Z",
"timestamp": 1764332692000
},
"deposited": {
"date-parts": [
[
2025,
11,
28
]
],
"date-time": "2025-11-28T12:24:53Z",
"timestamp": 1764332693000
},
"funder": [
{
"DOI": "10.13039/501100002920",
"award": [
"C2103-20G"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100002920",
"id-type": "DOI"
}
],
"name": "Research Grants Council, University Grants Committee"
},
{
"name": "Hong Kong Baptist University Seed Fund"
},
{
"name": "Hong Kong Baptist University Equipment Matching Fund"
}
],
"indexed": {
"date-parts": [
[
2025,
11,
28
]
],
"date-time": "2025-11-28T12:35:56Z",
"timestamp": 1764333356643,
"version": "3.46.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
11,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
11,
28
]
],
"date-time": "2025-11-28T00:00:00Z",
"timestamp": 1764288000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
11,
28
]
],
"date-time": "2025-11-28T00:00:00Z",
"timestamp": 1764288000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12964-025-02519-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12964-025-02519-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12964-025-02519-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
11,
28
]
]
},
"published-online": {
"date-parts": [
[
2025,
11,
28
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41586-024-07487-w",
"author": "J Abramson",
"doi-asserted-by": "publisher",
"first-page": "493",
"issue": "8016",
"journal-title": "Nature",
"key": "2519_CR1",
"unstructured": "Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630(8016):493–500. https://doi.org/10.1038/s41586-024-07487-w.",
"volume": "630",
"year": "2024"
},
{
"DOI": "10.1172/JCI148517",
"author": "JH Ahn",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Invest",
"key": "2519_CR2",
"unstructured": "Ahn JH, Kim JM, Hong SP, Choi SY, Yang MJ, Ju YS, et al. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest. 2021. https://doi.org/10.1172/JCI148517.",
"year": "2021"
},
{
"DOI": "10.1083/jcb.201607095",
"author": "HB Badgandi",
"doi-asserted-by": "publisher",
"first-page": "743",
"issue": "3",
"journal-title": "J Cell Biol",
"key": "2519_CR3",
"unstructured": "Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol. 2017;216(3):743–60. https://doi.org/10.1083/jcb.201607095.",
"volume": "216",
"year": "2017"
},
{
"DOI": "10.1016/j.jbc.2021.100306",
"author": "A Bayati",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "2519_CR4",
"unstructured": "Bayati A, Kumar R, Francis V, McPherson PS. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J Biol Chem. 2021. https://doi.org/10.1016/j.jbc.2021.100306.",
"year": "2021"
},
{
"DOI": "10.1111/jcmm.16729",
"author": "R Belgacemi",
"doi-asserted-by": "publisher",
"first-page": "7575",
"issue": "15",
"journal-title": "J Cell Mol Med",
"key": "2519_CR5",
"unstructured": "Belgacemi R, Diabasana Z, Hoarau A, Dubernard X, Mérol JC, Ruaux C, et al. Primary ciliogenesis is a crucial step for multiciliated cell determinism in the respiratory epithelium. J Cell Mol Med. 2021;25(15):7575–9. https://doi.org/10.1111/jcmm.16729.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1152/physiolgenomics.00015.2021",
"author": "R Buqaileh",
"doi-asserted-by": "publisher",
"first-page": "249",
"issue": "6",
"journal-title": "Physiol Genomics",
"key": "2519_CR6",
"unstructured": "Buqaileh R, Saternos H, Ley S, Aranda A, Forero K, Abou-Alaiwi WA. Can cilia provide an entry gateway for SARS-CoV-2 to human ciliated cells? Physiol Genomics. 2021;53(6):249–58. https://doi.org/10.1152/physiolgenomics.00015.2021.",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1016/j.tins.2022.11.003",
"author": "R Butowt",
"doi-asserted-by": "publisher",
"first-page": "75",
"issue": "1",
"journal-title": "Trends Neurosci",
"key": "2519_CR7",
"unstructured": "Butowt R, Bilinska K, von Bartheld CS. Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms. Trends Neurosci. 2023;46(1):75–90. https://doi.org/10.1016/j.tins.2022.11.003.",
"volume": "46",
"year": "2023"
},
{
"DOI": "10.1126/science.abd2985",
"author": "L Cantuti-Castelvetri",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "2519_CR8",
"unstructured": "Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020. https://doi.org/10.1126/science.abd2985.",
"year": "2020"
},
{
"DOI": "10.1126/science.abd3072",
"author": "JamesL Daly",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "2519_CR9",
"unstructured": "Daly James L, Simonetti Boris, Klein Katja, Chen Kai-En, Williamson Maia Kavanagh, Antón-Plágaro Carlos, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020. https://doi.org/10.1126/science.abd3072.",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.10.030",
"author": "Z Daniloski",
"doi-asserted-by": "publisher",
"first-page": "92",
"issue": "1",
"journal-title": "Cell",
"key": "2519_CR10",
"unstructured": "Daniloski Z, Jordan TX, Wessels HH, Hoagland DA, Kasela S, Legut M, et al. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell. 2021;184(1):92-105.e16. https://doi.org/10.1016/j.cell.2020.10.030.",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.celrep.2024.114164",
"doi-asserted-by": "publisher",
"key": "2519_CR11",
"unstructured": "Fagan RR, Lee DF, Geron M, Scherrer G, von Zastrow M, Ehrlich AT. Selective targeting of Mu opioid receptors to primary cilia. Cell Rep. 2024;43(5). https://doi.org/10.1016/j.celrep.2024.114164."
},
{
"DOI": "10.3389/fbioe.2023.1268782",
"author": "J Gonzalez-Rubio",
"doi-asserted-by": "publisher",
"journal-title": "Front Bioeng Biotechnol",
"key": "2519_CR12",
"unstructured": "Gonzalez-Rubio J, Le-Trilling VTK, Baumann L, Cheremkhina M, Kubiza H, Luengen AE, et al. SARS-CoV-2 particles promote airway epithelial differentiation and ciliation. Front Bioeng Biotechnol. 2023. https://doi.org/10.3389/fbioe.2023.1268782.",
"year": "2023"
},
{
"DOI": "10.1016/j.bbrc.2018.12.109",
"author": "S Han",
"doi-asserted-by": "publisher",
"first-page": "227",
"issue": "1",
"journal-title": "Biochem Biophys Res Commun",
"key": "2519_CR13",
"unstructured": "Han S, Miyoshi Ko, Shikada S, Amano G, Wang Y, Yoshimura T, et al. TULP3 is required for localization of membrane-associated proteins ARL13B and INPP5E to primary cilia. Biochem Biophys Res Commun. 2019;509(1):227–34. https://doi.org/10.1016/j.bbrc.2018.12.109.",
"volume": "509",
"year": "2019"
},
{
"DOI": "10.1016/j.cell.2022.11.010",
"author": "SJ Hesketh",
"doi-asserted-by": "publisher",
"first-page": "4971",
"issue": "26",
"journal-title": "Cell",
"key": "2519_CR14",
"unstructured": "Hesketh SJ, Mukhopadhyay AG, Nakamura D, Toropova K, Roberts AJ. IFT-a structure reveals carriages for membrane protein transport into cilia. Cell. 2022;185(26):4971-4985.e16. https://doi.org/10.1016/j.cell.2022.11.010.",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "2519_CR15",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.14348/molcells.2021.0082",
"author": "JJ Hong",
"doi-asserted-by": "publisher",
"first-page": "591",
"issue": "8",
"journal-title": "Mol Cells",
"key": "2519_CR16",
"unstructured": "Hong JJ, Kim KE, Park SY, Bok J, Seo JT, Moon SJ. Differential roles of Tubby family proteins in ciliary formation and trafficking. Mol Cells. 2021;44(8):591–601. https://doi.org/10.14348/molcells.2021.0082.",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1523/JNEUROSCI.3005-20.2021",
"author": "CHH Hor",
"doi-asserted-by": "publisher",
"first-page": "6850",
"issue": "32",
"journal-title": "J Neurosci",
"key": "2519_CR17",
"unstructured": "Hor CHH, Lo JCW, Cham ALS, Leong WY, Goh ELK. Multifaceted functions of Rab23 on primary cilium-mediated and Hedgehog signaling-mediated cerebellar granule cell proliferation. J Neurosci. 2021;41(32):6850–63. https://doi.org/10.1523/JNEUROSCI.3005-20.2021.",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1101/cshperspect.a021998",
"author": "H Ishikawa",
"doi-asserted-by": "publisher",
"journal-title": "Cold Spring Harb Perspect Biol",
"key": "2519_CR18",
"unstructured": "Ishikawa H, Marshall WF. Intraflagellar transport and ciliary dynamics. Cold Spring Harb Perspect Biol. 2017. https://doi.org/10.1101/cshperspect.a021998.",
"year": "2017"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"author": "CB Jackson",
"doi-asserted-by": "publisher",
"first-page": "3",
"issue": "1",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "2519_CR19",
"unstructured": "Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20. https://doi.org/10.1038/s41580-021-00418-x.",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1165/rcmb.2009-0328OC",
"author": "R Jain",
"doi-asserted-by": "publisher",
"first-page": "731",
"issue": "6",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "2519_CR20",
"unstructured": "Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, et al. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;43(6):731–9. https://doi.org/10.1165/rcmb.2009-0328OC.",
"volume": "43",
"year": "2010"
},
{
"DOI": "10.1038/s41598-022-11248-y",
"author": "V Lalioti",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Sci Rep",
"key": "2519_CR21",
"unstructured": "Lalioti V, González-Sanz S, Lois-Bermejo I, González-Jiménez P, Viedma-Poyatos Á, Merino A, et al. Cell surface detection of vimentin, ACE2 and SARS-CoV-2 spike proteins reveals selective colocalization at primary cilia. Sci Rep. 2022;12(1):7063. https://doi.org/10.1038/s41598-022-11248-y.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-19145-6",
"doi-asserted-by": "publisher",
"key": "2519_CR22",
"unstructured": "Lee IT, Nakayama T, Wu CT, Goltsev Y, Jiang S, Gall PA, et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat Commun. 2020;11(1). https://doi.org/10.1038/s41467-020-19145-6."
},
{
"DOI": "10.1371/journal.pgen.1011611",
"author": "WY Leong",
"doi-asserted-by": "publisher",
"issue": "8",
"journal-title": "PLoS Genet",
"key": "2519_CR23",
"unstructured": "Leong WY, Tung WL, Wilkie AOM, Hor CHH, Susan K. Dutcher. RAB23 loss-of-function mutation causes context-dependent ciliopathy in Carpenter syndrome. PLoS Genet. 2025;21(8):e1011611. https://doi.org/10.1371/journal.pgen.1011611.",
"volume": "21",
"year": "2025"
},
{
"DOI": "10.1111/febs.15491",
"author": "W Li",
"doi-asserted-by": "publisher",
"first-page": "3672",
"issue": "17",
"journal-title": "FEBS J",
"key": "2519_CR24",
"unstructured": "Li W, Li M, Ou G. COVID-19, Cilia, and Smell. FEBS J. 2020;287(17):3672–6. https://doi.org/10.1111/febs.15491.",
"volume": "287",
"year": "2020"
},
{
"DOI": "10.15252/msb.202110396",
"author": "X Liu",
"doi-asserted-by": "publisher",
"journal-title": "Mol Syst Biol",
"key": "2519_CR25",
"unstructured": "Liu X, Huuskonen S, Laitinen T, Redchuk T, Bogacheva M, Salokas K, et al. SARS-CoV-2–host proteome interactions for antiviral drug discovery. Mol Syst Biol. 2021. https://doi.org/10.15252/msb.202110396.",
"year": "2021"
},
{
"DOI": "10.1042/CS20200899",
"author": "L Lubbe",
"doi-asserted-by": "publisher",
"first-page": "2851",
"issue": "21",
"journal-title": "Clin Sci",
"key": "2519_CR26",
"unstructured": "Lubbe L, Cozier GE, Delia Oosthuizen K, Acharya R, Sturrock ED. ACE2 and ACE: Structure-Based Insights into Mechanism, Regulation and Receptor Recognition by SARS-CoV. Clin Sci. 2020;134(21):2851–71. https://doi.org/10.1042/CS20200899.",
"volume": "134",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00791-2022",
"author": "E Luczka-Majérus",
"doi-asserted-by": "publisher",
"journal-title": "Eur Respir J",
"key": "2519_CR27",
"unstructured": "Luczka-Majérus E, Bonnomet A, Germain A, Lalun N, Kileztky C, Perotin JM, et al. Ciliogenesis is intrinsically altered in COPD small airways. Eur Respir J. 2022. https://doi.org/10.1183/13993003.00791-2022.",
"year": "2022"
},
{
"DOI": "10.1091/mbc.E16-07-0505",
"author": "S Mukhopadhyay",
"doi-asserted-by": "publisher",
"first-page": "233",
"issue": "2",
"journal-title": "Mol Biol Cell",
"key": "2519_CR28",
"unstructured": "Mukhopadhyay S, Badgandi HB, Hwang SH, Somatilaka B, Shimada IS, Pal K. Trafficking to the primary cilium membrane. Mol Biol Cell. 2017;28(2):233–9. https://doi.org/10.1091/mbc.E16-07-0505.",
"volume": "28",
"year": "2017"
},
{
"DOI": "10.1186/gb-2011-12-6-225",
"author": "S Mukhopadhyay",
"doi-asserted-by": "publisher",
"journal-title": "Genome Biol",
"key": "2519_CR29",
"unstructured": "Mukhopadhyay S, Jackson PK. The tubby family proteins. Genome Biol. 2011. https://doi.org/10.1186/gb-2011-12-6-225.",
"year": "2011"
},
{
"DOI": "10.1101/GAD.1966210",
"author": "S Mukhopadhyay",
"doi-asserted-by": "publisher",
"first-page": "2180",
"issue": "19",
"journal-title": "Genes Dev",
"key": "2519_CR30",
"unstructured": "Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010;24(19):2180–93. https://doi.org/10.1101/GAD.1966210.",
"volume": "24",
"year": "2010"
},
{
"DOI": "10.7759/CUREUS.13881",
"doi-asserted-by": "publisher",
"key": "2519_CR31",
"unstructured": "Oke, Ibiyemi O, Olubunmi O Oladunjoye, Adeolu O Oladunjoye, Anish Paudel, and Ryan Zimmerman. “Bell’s palsy as a late neurologic manifestation of COVID-19 infection.” Cureus. 2021;13(3). https://doi.org/10.7759/CUREUS.13881."
},
{
"DOI": "10.1002/pro.3978",
"author": "R Oughtred",
"doi-asserted-by": "publisher",
"first-page": "187",
"issue": "1",
"journal-title": "Protein Sci",
"key": "2519_CR32",
"unstructured": "Oughtred R, Rust J, Chang C, Breitkreutz BJ, Stark C, Willems A, et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021;30(1):187–200. https://doi.org/10.1002/pro.3978.",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1016/j.isci.2023.106274",
"author": "D Pal",
"doi-asserted-by": "publisher",
"journal-title": "iScience",
"key": "2519_CR33",
"unstructured": "Pal D, De K, Yates TB, Kolape J, Muchero W. Mutating novel interaction sites in NRP1 reduces SARS-CoV-2 spike protein internalization. iScience. 2023. https://doi.org/10.1016/j.isci.2023.106274.",
"year": "2023"
},
{
"DOI": "10.1091/mbc.E24-09-0426",
"author": "VR Palicharla",
"doi-asserted-by": "publisher",
"journal-title": "Mol Biol Cell",
"key": "2519_CR34",
"unstructured": "Palicharla VR, Badgandi HB, Hwang SH, Legué E, Liem KF, Mukhopadhyay S. A defined tubby domain β-barrel surface region of TULP3 mediates ciliary trafficking of diverse cargoes. Mol Biol Cell. 2025. https://doi.org/10.1091/mbc.E24-09-0426.",
"year": "2025"
},
{
"DOI": "10.1091/mbc.E22-10-0473",
"doi-asserted-by": "publisher",
"key": "2519_CR35",
"unstructured": "Palicharla VR, Hwang SH, Somatilaka BN, Legué E, Shimada IS, Familiari NE, et al. Interactions between TULP3 tubby domain and ARL13B amphipathic helix promote lipidated protein transport to cilia. Mol Biol Cell. 2023(3). https://doi.org/10.1091/mbc.E22-10-0473."
},
{
"DOI": "10.1183/13993003.00122-2018",
"author": "J-M Perotin",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "2519_CR36",
"unstructured": "Perotin J-M, Coraux C, Lagonotte E, Birembaut PL, Delepine G, Polette MC, et al. Alteration of primary cilia in COPD. Eur Respir J. 2018;52(1):1800122. https://doi.org/10.1183/13993003.00122-2018.",
"volume": "52",
"year": "2018"
},
{
"DOI": "10.1074/jbc.M117.783845",
"author": "JM Pinskey",
"doi-asserted-by": "publisher",
"first-page": "15192",
"issue": "37",
"journal-title": "J Biol Chem",
"key": "2519_CR37",
"unstructured": "Pinskey JM, Franks NE, McMellen AN, Giger RJ, Allen BL. Neuropilin-1 promotes Hedgehog signaling through a novel cytoplasmic motif. J Biol Chem. 2017;292(37):15192–204. https://doi.org/10.1074/jbc.M117.783845.",
"volume": "292",
"year": "2017"
},
{
"DOI": "10.1038/nrm.2017.60",
"author": "JF Reiter",
"doi-asserted-by": "publisher",
"first-page": "533",
"issue": "9",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "2519_CR38",
"unstructured": "Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol. 2017;18(9):533–47. https://doi.org/10.1038/nrm.2017.60.",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1038/s41467-021-24521-x",
"author": "R Robinot",
"doi-asserted-by": "publisher",
"journal-title": "Nat Commun",
"key": "2519_CR39",
"unstructured": "Robinot R, Hubert M, de Melo GD, Lazarini F, Bruel T, Smith N, et al. SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat Commun. 2021. https://doi.org/10.1038/s41467-021-24521-x.",
"year": "2021"
},
{
"DOI": "10.1016/J.NBD.2022.105715",
"author": "MG Savelieff",
"doi-asserted-by": "publisher",
"journal-title": "Neurobiol Dis",
"key": "2519_CR40",
"unstructured": "Savelieff MG, Feldman EL, Stino AM. Neurological sequela and disruption of neuron-glia homeostasis in SARS-CoV-2 infection. Neurobiol Dis. 2022;168:105715. https://doi.org/10.1016/J.NBD.2022.105715.",
"volume": "168",
"year": "2022"
},
{
"DOI": "10.3390/ijms23095124",
"author": "T Schreiner",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "2519_CR41",
"unstructured": "Schreiner T, Allnoch L, Beythien G, Marek K, Becker K, Schaudien D, et al. SARS-CoV-2 infection dysregulates cilia and basal cell homeostasis in the respiratory epithelium of hamsters. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23095124.",
"year": "2022"
},
{
"DOI": "10.1126/science.1124534",
"author": "V Singla",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "2519_CR42",
"unstructured": "Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006. https://doi.org/10.1126/science.1124534.",
"year": "2006"
},
{
"DOI": "10.1038/s41392-023-01387-7",
"author": "P Su",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "2519_CR43",
"unstructured": "Su P, Zhou F, Zhang L. Motile cilia and microvillar: accomplices of SARS-CoV-2 in penetrating mucus barrier and infecting airway epithelium. Signal Transduct Target Ther. 2023;8(1):117. https://doi.org/10.1038/s41392-023-01387-7.",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1016/s2215-0366(22)00260-7",
"author": "M Taquet",
"doi-asserted-by": "publisher",
"first-page": "815",
"issue": "10",
"journal-title": "Lancet Psychiatry",
"key": "2519_CR44",
"unstructured": "Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815–27. https://doi.org/10.1016/s2215-0366(22)00260-7.",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1146/annurev-physiol-021014-071931",
"author": "AE Tilley",
"doi-asserted-by": "publisher",
"first-page": "379",
"journal-title": "Annu Rev Physiol",
"key": "2519_CR45",
"unstructured": "Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379–406. https://doi.org/10.1146/annurev-physiol-021014-071931.",
"volume": "77",
"year": "2015"
},
{
"DOI": "10.1126/sciadv.abq6527",
"author": "JA Torchia",
"doi-asserted-by": "publisher",
"journal-title": "Sci Adv",
"key": "2519_CR46",
"unstructured": "Torchia JA, Tavares AH, Carstensen LS, Chen DY, Huang J, Xiao T, et al. Optimized ACE2 decoys neutralize antibody-resistant SARS-CoV-2 variants through functional receptor mimicry and treat infection in vivo. Sci Adv. 2022. https://doi.org/10.1126/sciadv.abq6527.",
"year": "2022"
},
{
"DOI": "10.1126/SCITRANSLMED.ADI2623",
"author": "E Urano",
"doi-asserted-by": "publisher",
"first-page": "293",
"issue": "711",
"journal-title": "Science Translational Medicine",
"key": "2519_CR47",
"unstructured": "Urano E, Itoh Y, Suzuki T, Sasaki T, Kishikawa JI, Akamatsu K, et al. An Inhaled ACE2 Decoy Confers Protection against SARS-CoV-2 Infection in Preclinical Models. Science Translational Medicine. 2023;15(711):293. https://doi.org/10.1126/SCITRANSLMED.ADI2623.",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0216705",
"author": "TJP Van Dam",
"doi-asserted-by": "publisher",
"journal-title": "PLoS One",
"key": "2519_CR48",
"unstructured": "Van Dam TJP, Kennedy J, van der Lee R, de Vrieze E, Wunderlich KA, Rix S, et al. Ciliacarta: an integrated and validated compendium of ciliary genes. PLoS One. 2019. https://doi.org/10.1371/journal.pone.0216705.",
"year": "2019"
},
{
"DOI": "10.1186/2046-2530-2-7",
"author": "TJP Van Dam",
"doi-asserted-by": "publisher",
"journal-title": "Cilia",
"key": "2519_CR49",
"unstructured": "Van Dam TJP, Wheway G, Slaats GG, Huynen MA, Giles RH. The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia. 2013. https://doi.org/10.1186/2046-2530-2-7.",
"year": "2013"
},
{
"DOI": "10.1038/s41556-021-00796-6",
"author": "Si Wang",
"doi-asserted-by": "publisher",
"first-page": "1314",
"issue": "12",
"journal-title": "Nat Cell Biol",
"key": "2519_CR50",
"unstructured": "Wang Si, Yao X, Ma S, Ping Y, Fan Y, Sun S, et al. A single-cell transcriptomic landscape of the lungs of patients with COVID-19. Nat Cell Biol. 2021;23(12):1314–28. https://doi.org/10.1038/s41556-021-00796-6.",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2022.11.030",
"author": "CT Wu",
"doi-asserted-by": "publisher",
"first-page": "112",
"issue": "1",
"journal-title": "Cell",
"key": "2519_CR51",
"unstructured": "Wu CT, Lidsky PV, Xiao Y, Cheng R, Lee IT, Nakayama T, et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell. 2023;186(1):112-130.e20. https://doi.org/10.1016/j.cell.2022.11.030.",
"volume": "186",
"year": "2023"
},
{
"DOI": "10.1002/JMV.27244",
"author": "F Zhang",
"doi-asserted-by": "publisher",
"first-page": "6671",
"issue": "12",
"journal-title": "J Med Virol",
"key": "2519_CR52",
"unstructured": "Zhang F, Li W, Feng J, Ramos S, da Silva E, Zhang JH, et al. SARS-CoV-2 Pseudovirus Infectivity and Expression of Viral Entry-Related Factors ACE2, TMPRSS2, Kim-1, and NRP-1 in Human Cells from the Respiratory, Urinary, Digestive, Reproductive, and Immune Systems. J Med Virol. 2021;93(12):6671–85. https://doi.org/10.1002/JMV.27244.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26139",
"author": "MW Zhuang",
"doi-asserted-by": "publisher",
"first-page": "2693",
"issue": "11",
"journal-title": "J Med Virol",
"key": "2519_CR53",
"unstructured": "Zhuang MW, Cheng Y, Zhang J, Jiang XM, Wang Li, Deng J, et al. Increasing host cellular receptor—angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-NCoV (or SARS-CoV-2) infection. J Med Virol. 2020;92(11):2693–701. https://doi.org/10.1002/jmv.26139.",
"volume": "92",
"year": "2020"
}
],
"reference-count": 53,
"references-count": 53,
"relation": {},
"resource": {
"primary": {
"URL": "https://biosignaling.biomedcentral.com/articles/10.1186/s12964-025-02519-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Primary cilium and TULP3-dependent ciliary targeting of ACE2 in SARS-CoV-2 tropism",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "23"
}