Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: preliminary results of a randomized double-blind clinical trial
et al., Epidemiology and Health, doi:10.4178/epih.e2021032, May 2021
24th treatment shown to reduce risk in
May 2021, now with p = 0.024 from 8 studies.
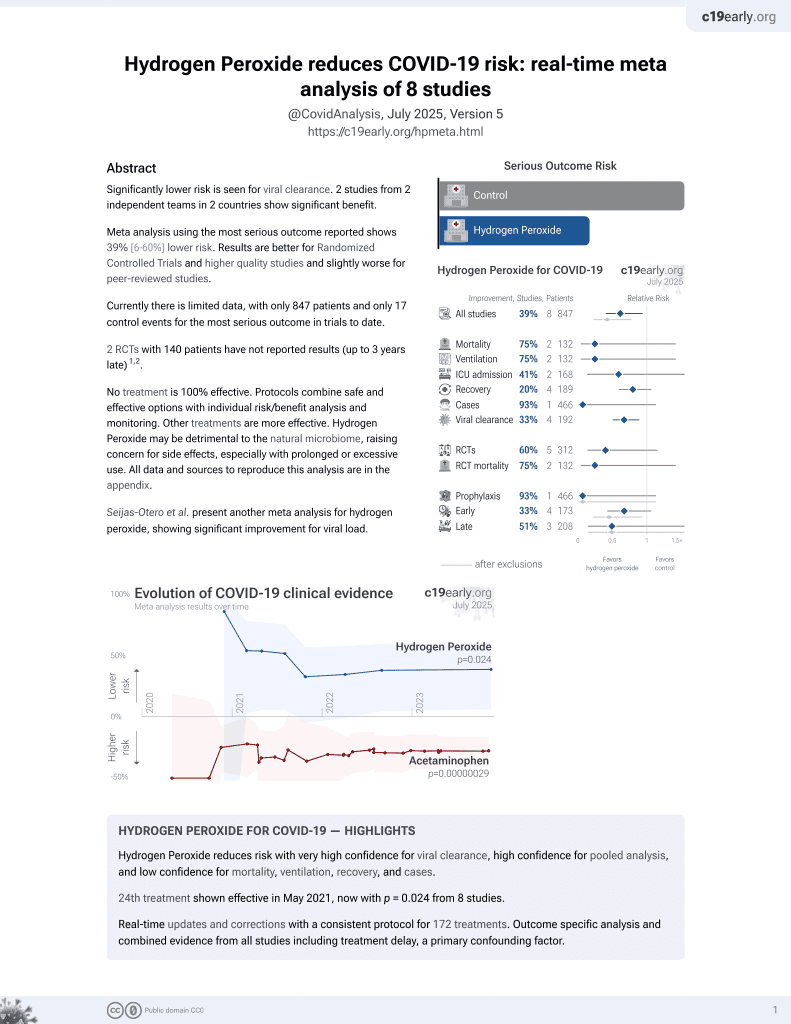
Lower risk for viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT very late treatment (>10 days from onset) comparing hydrogen peroxide + mint essence with water + mint essence, showing no significant differences.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
|
risk of ICU admission, 50.0% lower, RR 0.50, p = 1.00, treatment 1 of 20 (5.0%), control 2 of 20 (10.0%), NNT 20.
|
|
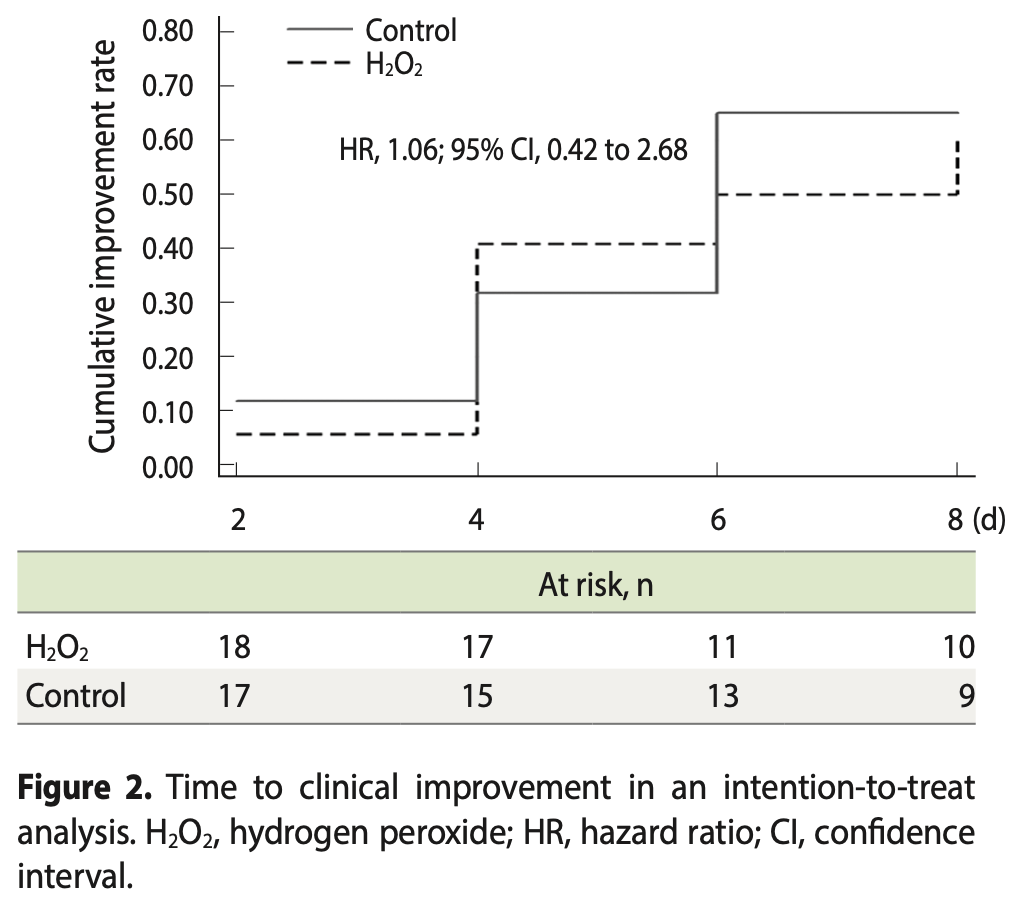
improvement, 5.7% lower, HR 0.94, p = 0.91, treatment 18, control 17, inverted to make HR<1 favor treatment.
|
|
time to discharge, 7.0% lower, relative time 0.93, p = 0.61, treatment mean 3.86 (±1.6) n=18, control mean 4.15 (±1.77) n=17.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Di Domênico et al., 1 May 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, survey, 9 authors, average treatment delay 10.72 days.
Contact: pedrocorazza@upf.br.
Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: preliminary results of a randomized double-blind clinical trial
Epidemiology and Health, doi:10.4178/epih.e2021032
OBJECTIVES: To evaluate the effectiveness of hydrogen peroxide (H2O2) in the form of mouthwash and nasal spray as an auxiliary treatment for coronavirus disease 2019 .
METHODS: Forty hospitalized patients who tested positive for severe acute respiratory syndrome coronavirus 2 using a reverse-transcription polymerase chain reaction test were evaluated. They were randomly divided into an experimental group (n = 20; gargling with 1.0% H2O2 and nasal wash with 0.5% H2O2) or a control group (n = 20). The solutions were used for 7 days and the patients were monitored every 2 days, for a total of 8 days. At check-ups, patients were asked about their symptoms and possible adverse effects of the solutions. The presence and severity (mild, moderate, or severe) of symptoms were recorded. Data were compared using the Student test and the Fisher exact test (α = 0.05).
RESULTS: There was no significant difference between the 2 groups in the length of hospital stay (p = 0.65). The most frequent symptom on day 0 was coughing (72.0% in the experimental group and 76.5% in the control group), which abated over time. There was no significant difference between the groups in the evaluated symptoms. Most (75.0%) of the patients in the experimental group presented a reduction in dyspnea between days 0 and 2. Few patients reported adverse effects from the use of the solutions. CONCLUSIONS: H2O2 as a mouthwash and nasal spray is safe to use. There is insufficient evidence to demonstrate that H2O2 is effective as an auxiliary treatment for hospitalized COVID-19 patients.
CONFLICT OF INTEREST The authors have no conflicts of interest to declare for this study.
AUTHOR CONTRIBUTIONS Conceptualization: PHC, MBDD. Data curation: KC. Formal analysis: KC. Funding acquisition: MBDD. Methodology: HC, THJP, RBS, UL, VPA, VWG. Project administration: PHC, MBDD, HC. Writing -original draft: MBDD, PHC, HC. Writing -review & editing: KC, THJP, RBS, UL, VPA, VWG, KC.
References
Amorim Dos Santos, Normando, Da Silva, Paula, Cembranel, Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations?, Int J Infect Dis
Boyd, Effects on gingivitis of daily rinsing with 1.5% H2O2, J Clin Periodontol
Byon, Heath, Chen, Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells, Redox Biol
Caruso, Prete, Lazzarino, Hydrogen peroxide and viral infections: a literature review with research hypothesis definition in relation to the current COVID-19 pandemic, Med Hypotheses
Cheng, Wong, Kwan, Hui, Yuen, Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2, J Hosp Infect
Docherty, Harrison, Green, Hardwick, Pius et al., Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ
Favia, Tempesta, Barile, Brienza, Capodiferro et al., COVID-19 symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the University Hospital Policlinic of Bari with a purpose of a new classification, J Clin Med
Garg, Kim, Whitaker, 'halloran, Cummings et al., Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 -COVID-NET, 14 States, MMWR Morb Mortal Wkly Rep
Gottsauner, Michaelides, Schmidt, Scholz, Buchalla et al., A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clin Oral Investig
Grant, Geoghegan, Arbyn, Mohammed, Mcguinness, The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries, PLoS One
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy, JAMA
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Hathway, COVID tongue, Br Dent J
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clin Oral Investig
Hu, Sun, Dai, Deng, Li et al., Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis, J Clin Virol
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Lancet
Kampf, Todt, Pfaender, Steinmann, Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents, J Hosp Infect
Lauer, Grantz, Bi, Jones, Zheng et al., The incubation period of coronavirus disease 2019 (COV-ID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med
Li, Pei, Chen, Song, Zhang et al., Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2), Science
Lu, Liu, Jia, 2019-nCoV transmission through the ocular surface must not be ignored, Lancet
Marshall, Cancro, Fischman, Hydrogen peroxide: a review of its use in dentistry, J Periodontol
O'donnell, Thomas, Stanton, Maillard, Murphy et al., Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function (Oxf)
Omidbakhsh, Sattar, Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant, Am J Infect Control
Peng, Xu, Li, Cheng, Zhou et al., Transmission routes of 2019-nCoV and controls in dental practice, Int J Oral Sci
Riad, Kassem, Hockova, Badrah, Klugar, Tongue ulcers associated with SARS-CoV-2 infection: a case series, Oral Dis, doi:10.1111/odi.13635
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA
Sharma, Acharya, Verma, Singhal, Singla, Efficacy of chlorhexidine, hydrogen peroxide and tulsi extract mouthwash in reducing halitosis using spectrophotometric analysis: a randomized controlled trial, J Clin Exp Dent
To, Tsang, Leung, Tam, Wu et al., Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, Lancet Infect Dis
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID-2019, Nature
Yang, Zheng, Gou, Pu, Chen et al., Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis, Int J Infect Dis
Zheng, Peng, Xu, Zhao, Liu et al., Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis, J Infect
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.4178/epih.e2021032",
"ISSN": [
"2092-7193"
],
"URL": "http://dx.doi.org/10.4178/epih.e2021032",
"abstract": "<jats:p>OBJECTIVES: To evaluate the effectiveness of hydrogen peroxide (H2O2) in the form of mouthwash and nasal spray as an auxiliary treatment for coronavirus disease 2019 (COVID-19).METHODS: Forty hospitalized patients who tested positive for severe acute respiratory syndrome coronavirus 2 using a reverse-transcription polymerase chain reaction test were evaluated. They were randomly divided into an experimental group (n= 20; gargling with 1.0% H2O2 and nasal wash with 0.5% H2O2) or a control group (n= 20). The solutions were used for 7 days and the patients were monitored every 2 days, for a total of 8 days. At check-ups, patients were asked about their symptoms and possible adverse effects of the solutions. The presence and severity (mild, moderate, or severe) of symptoms were recorded. Data were compared using the Student test and the Fisher exact test (α= 0.05).RESULTS: There was no significant difference between the 2 groups in the length of hospital stay (p= 0.65). The most frequent symptom on day 0 was coughing (72.0% in the experimental group and 76.5% in the control group), which abated over time. There was no significant difference between the groups in the evaluated symptoms. Most (75.0%) of the patients in the experimental group presented a reduction in dyspnea between days 0 and 2. Few patients reported adverse effects from the use of the solutions.CONCLUSIONS: H2O2 as a mouthwash and nasal spray is safe to use. There is insufficient evidence to demonstrate that H2O2 is effective as an auxiliary treatment for hospitalized COVID-19 patients.</jats:p>",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-01-20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-05-01"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-05-01"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2166-3052",
"affiliation": [],
"authenticated-orcid": true,
"family": "Di Domênico",
"given": "Marielle Bazzo",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-2751-0329",
"affiliation": [],
"authenticated-orcid": true,
"family": "Cesca",
"given": "Henrique",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3699-4225",
"affiliation": [],
"authenticated-orcid": true,
"family": "Ponciano",
"given": "Thales Henrique Jincziwski",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8188-480X",
"affiliation": [],
"authenticated-orcid": true,
"family": "dos Santos",
"given": "Renan Brandenburg",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7382-632X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Lenz",
"given": "Ulysses",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3059-5095",
"affiliation": [],
"authenticated-orcid": true,
"family": "Antunes",
"given": "Vinícius Picoli",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9897-0333",
"affiliation": [],
"authenticated-orcid": true,
"family": "Godinho",
"given": "Vinicius Webber",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7276-1074",
"affiliation": [],
"authenticated-orcid": true,
"family": "Collares",
"given": "Kauê",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9480-1607",
"affiliation": [],
"authenticated-orcid": true,
"family": "Corazza",
"given": "Pedro Henrique",
"sequence": "additional"
}
],
"container-title": "Epidemiology and Health",
"container-title-short": "Epidemiol Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"e-epih.org"
]
},
"created": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T15:33:00Z",
"timestamp": 1619883180000
},
"deposited": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T02:08:23Z",
"timestamp": 1661306903000
},
"funder": [
{
"DOI": "10.13039/501100002322",
"doi-asserted-by": "crossref",
"name": "Coordination for the Improvement of Higher Education Personnel"
}
],
"indexed": {
"date-parts": [
[
2023,
6,
13
]
],
"date-time": "2023-06-13T09:27:02Z",
"timestamp": 1686648422356
},
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2021,
5,
1
]
]
},
"language": "en",
"link": [
{
"URL": "http://e-epih.org/upload/pdf/epih-43-e2021032.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2679",
"original-title": [],
"page": "e2021032",
"prefix": "10.4178",
"published": {
"date-parts": [
[
2021,
5,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
1
]
]
},
"publisher": "Korean Society of Epidemiology",
"reference": [
{
"DOI": "10.1016/s0140-6736(20)30313-5",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1126/science.abb3221",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"author": "Guan",
"first-page": "1708",
"key": "ref3",
"volume-title": "Clinical characteristics of coronavirus disease 2019 in China",
"year": "2020"
},
{
"author": "Lauer",
"first-page": "577",
"key": "ref4",
"volume-title": "The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6915e3",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1371/journal.pone.0234765",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1136/bmj.m1985",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"author": "Grasselli",
"first-page": "1574",
"key": "ref8",
"volume-title": "Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy",
"year": "2020"
},
{
"DOI": "10.1016/s0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1001/jama.2020.12839",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/s1473-3099(20)30196-1",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1038/s41368-020-0075-9",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1056/nejmc2001737",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1007/s00784-020-03413-2",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.ajic.2005.06.002",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1016/j.jhin.2020.01.022",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/j.jhin.2020.04.003",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.4317/jced.55523",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1902/jop.1995.66.9.786",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"author": "Boyd",
"first-page": "557",
"key": "ref22",
"volume-title": "Effects on gingivitis of daily rinsing with 1.5% H2O2",
"year": "1989"
},
{
"DOI": "10.1016/j.mehy.2020.109910",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.redox.2016.08.015",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.jcv.2020.104371",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/j.ijid.2020.03.017",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/j.jinf.2020.04.021",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.3390/jcm10040757",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1016/j.ijid.2020.06.012",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1038/s41415-021-2666-z",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1111/odi.13635",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1093/function/zqaa002",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"doi-asserted-by": "publisher",
"key": "ref33"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "http://e-epih.org/journal/view.php?doi=10.4178/epih.e2021032"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: preliminary results of a randomized double-blind clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.4178/crossmark_policy",
"volume": "43"
}