Diacerein is an oral, small molecule anti-inflammatory drug that inhibits the interleukin-1β (IL-1β) pathway and inflammasome activation.
Recent:Carmo.
Oct 7 2024 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2024.1402032 | Diacerein reduces inflammasome activation and SARS-CoV-2 virus replication: a proof-of-concept translational study |
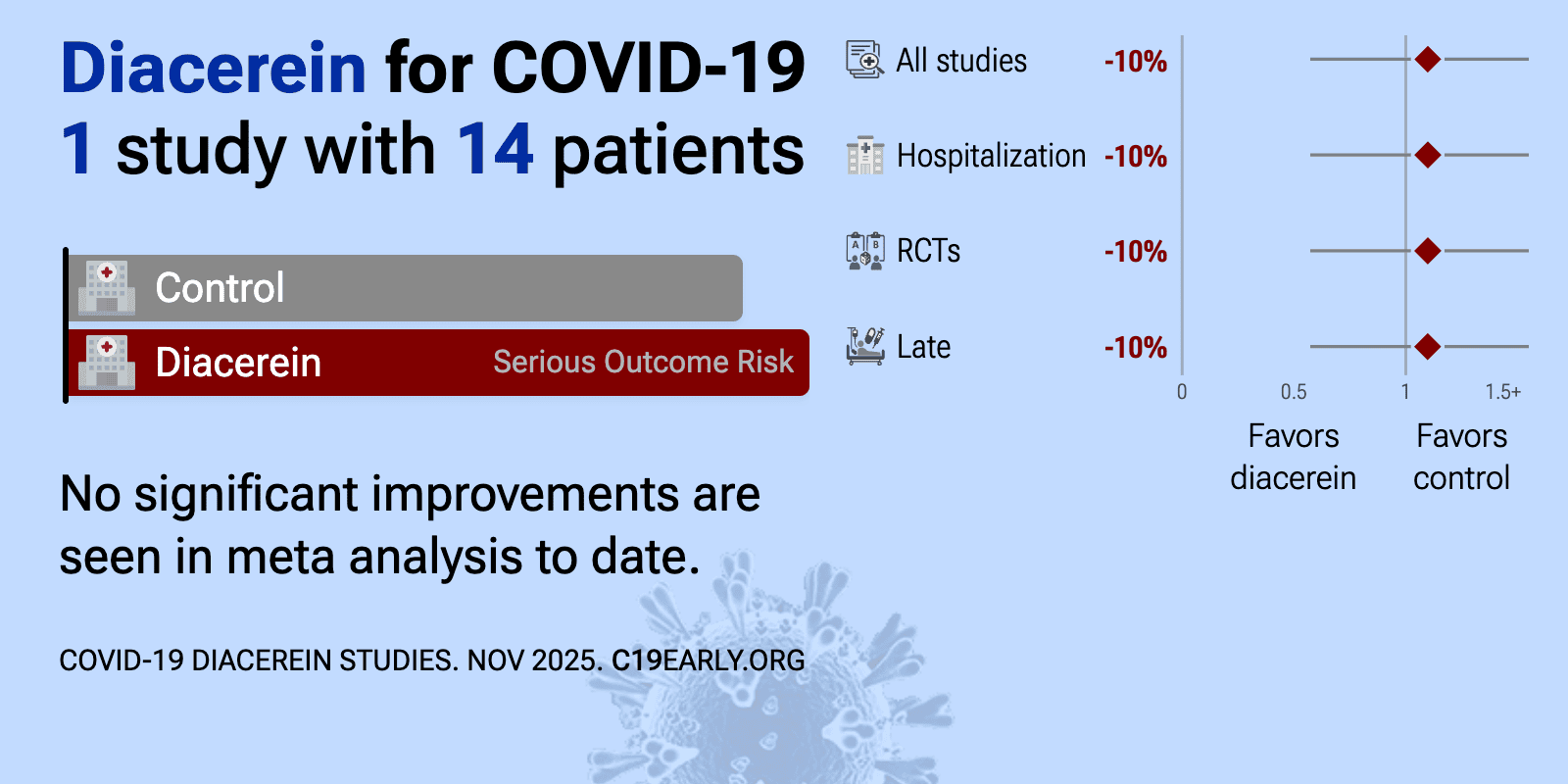
| 10% longer hospitalization (p=0.79). RCT 14 hospitalized patients with mild to moderate COVID-19 showing reduced plasma inflammasome markers with diacerein treatment, but no significant difference in duration of hospitalization. The study was halted early due to declining CO.. | ||