Cochrane's COVID‐19 Living Systematic Reviews: A Mixed‐Methods Study of Their Conduct, Reporting and Currency
et al., Cochrane Evidence Synthesis and Methods, doi:10.1002/cesm.70024, Mar 2025
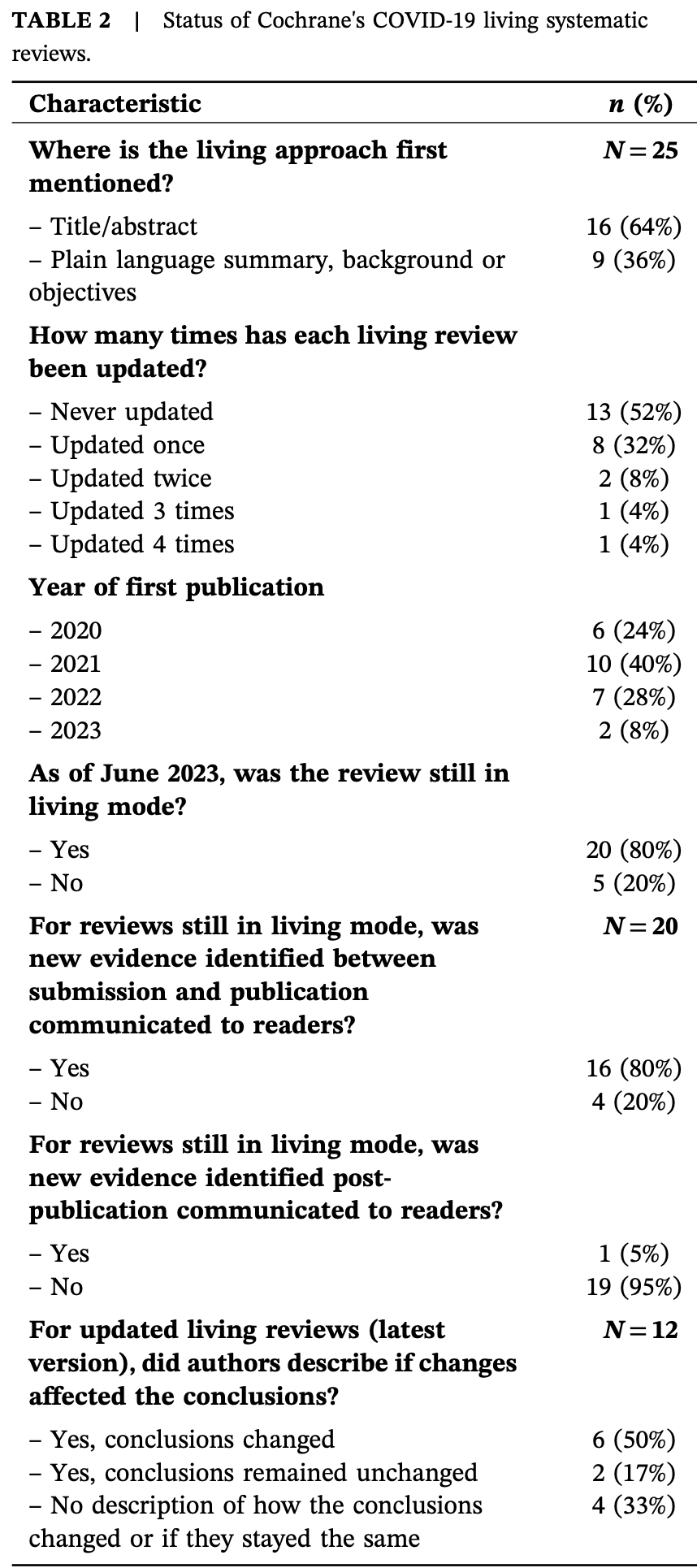
Analysis of 25 Cochrane COVID-19 living systematic reviews, showing that they were rarely updated and are significantly out of date. Despite being designed for rapid incorporation of new evidence, 52% were never updated, 32% were updated once, and only 16% were updated more than once. Authors identified 119 potentially eligible studies not yet included in the reviews. Interviews with six authors revealed barriers including editorial delays, loss of funding, waning commitment as the pandemic evolved, and screening burden. Only one review successfully maintained a transparent system for reporting new evidence between published versions.
De Silva et al., 28 Mar 2025, peer-reviewed, 3 authors.
Contact: kevindu.desilva@monash.edu.
Cochrane's COVID‐19 Living Systematic Reviews: A Mixed‐Methods Study of Their Conduct, Reporting and Currency
Cochrane Evidence Synthesis and Methods, doi:10.1002/cesm.70024
Background: Living systematic reviews (LSRs) should provide up-to-date evidence for priority questions where the evidence may be uncertain and fast-moving. LSRs featured prominently during COVID-19 and formed part of Cochrane's response to the pandemic. We conducted a mixed-methods study to describe the characteristics of Cochrane's COVID-19 living reviews, determine the currency of the included evidence, and evaluate authors' experiences in conducting and publishing these reviews. Methods: We identified living reviews of COVID-19 from the Cochrane Database of Systematic Reviews and extracted data on the number of versions published and publication timelines. We assessed the currency of evidence by comparing studies included in the reviews against a comprehensive list of studies maintained for the Australian living guidelines for COVID-19. The qualitative component involved semi-structured interviews with review authors to identify the barriers and enablers to conducting, reporting and publishing living reviews. Findings: Cochrane published 25 COVID-19 living systematic reviews. Half of these reviews had not been updated when assessed in June 2023 and only four had been updated more than once. A total of 118 studies were included in the living reviews. We estimated that an additional 119 studies were available and potentially relevant for inclusion. Interviews with six authors indicated that publication timelines were reduced by editorial delays, loss of funding, waning commitment, and the burden of screening search results. An inability to communicate the living status of reviews in the Cochrane Library was a common frustration for many authors. Although authors felt the conclusions of their reviews were still current, only one living review communicated its updated status and made new evidence accessible after the review was published.
Author Contributions Kevindu De Silva: conceptualization, data curation, formal analysis, investigation, methodology, writingoriginal draft, writingreview and editing. Tari Turner: conceptualization, formal analysis, investigation, methodology, supervision, validation, writingreview and editing. Steve McDonald: conceptualization, formal analysis, investigation, methodology, supervision, validation, writingreview and editing.
Ethics Statement Ethics approval was provided by Monash University Human Research Ethics Committee (Project ID: 37953).
Conflicts of Interest TT and SM developed Cochrane's guidance for living systematic reviews, evaluated Cochrane's pre-pandemic LSR pilot, and are co-authors of Cochrane living reviews unrelated to COVID-19. Both have Cochrane editorial roles -TT is a member of the Cochrane Library Editorial Board and SM is a member of the Cochrane search peer review panel. All authors are associated with the Australian Living Evidence Collaboration.
Supporting Information Additional supporting information can be found online in the Supporting Information section.
References
Akl, Khabsa, Iannizzi, Extension of the PRISMA 2020 Statement for Living Systematic Reviews (Prisma-Lsr): Checklist and Explanation, BMJ
Ansems, Grundeis, Dahms, Remdesivir for the Treatment of COVID-19, Cochrane Database of Systematic Reviews
Butler, Hartmann-Boyce, Livingstone-Banks, Turner, Lindson, Optimizing Process and Methods for a Living Systematic Review: 30 Search Updates and Three Review Updates Later, Journal of Clinical Epidemiology
Chen, Luo, Li, Characteristics of Living Systematic Review for COVID-19, Clinical Epidemiology
Chou, Dana, Jungbauer, Update Alert 8: Masks for Prevention of Respiratory Virus Infections, Including SARS-CoV-2, in Health Care and Community Settings, Annals of Internal Medicine
Chou, Dana, Major Update: Masks for Prevention of SARS-CoV-2 in Health Care and Community Settings-Final Update of a Living, Rapid Review, Annals of Internal Medicine
Cohen, Edelman, Paynter, Henderson, Risk of Thromboembolism in Patients With COVID-19 who Are Using Hormonal Contraception, Cochrane Database of Systematic Reviews
Davidson, Menon, Chaimani, Interleukin-1 Blocking Agents for Treating COVID-19, Cochrane Database of Systematic Reviews
Deeks, Dinnes, Takwoingi, Antibody Tests for Identification of Current and Past infection With SARS-CoV-2, Cochrane Database of Systematic Reviews
Dinnes, Deeks, Adriano, Rapid, Point-of-Care Antigen and Molecular-Based Tests for Diagnosis of SARS-CoV-2 Infection, Cochrane Database of Systematic Reviews
Elliott, Synnot, Turner, Living Systematic Review: 1. Introduction-the Why, What, When, and How, Journal of Clinical Epidemiology
Elliott, Turner, Clavisi, Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice gap, PLoS Medicine
Ghosn, Chaimani, Evrenoglou, Interleukin-6 Blocking Agents for Treating COVID-19: A Living Systematic Review, Cochrane Database of Systematic Reviews
Graña, Ghosn, Evrenoglou, Efficacy and Safety of COVID-19 Vaccines, Cochrane Database of Systematic Reviews
Griesel, Wagner, Mikolajewska, Inhaled Corticosteroids for the Treatment of COVID-19, Cochrane Database of Systematic Reviews
Heron, Buitrago-Garcia, Ipekci, How to Update a Living Systematic Review and Keep It Alive During a Pandemic: A Practical Guide, Systematic Reviews
Hirsch, Park, Piechotta, SARS-CoV-2-Neutralising Monoclonal Antibodies to Prevent COVID-19, Cochrane Database of Systematic Reviews
Hoffmann, Allers, Rombey, Nearly 80 Systematic Reviews Were Published Each Day: Observational Study on Trends in Epidemiology and Reporting Over the Years 2000-2019, Journal of Clinical Epidemiology
Iannizzi, Dorando, Burns, Methodological Challenges for Living Systematic Reviews Conducted During the COVID-19 Pandemic: A Concept Paper, Journal of Clinical Epidemiology
Kimber, Valk, Chai, Hyperimmune Immunoglobulin for People With COVID-19, Cochrane Database of Systematic Reviews
Kramer, Prinz, Fichtner, Janus Kinase Inhibitors for the Treatment of COVID-19, Cochrane Database of Systematic Reviews
Kreuzberger, Hirsch, Chai, SARS-CoV-2-Neutralising Monoclonal Antibodies for Treatment of COVID-19, Cochrane Database of Systematic Reviews
Lindson, Butler, Mcrobbie, Electronic Cigarettes for Smoking Cessation, Cochrane Database of Systematic Reviews
Luo, Chen, Liu, Methodological Quality and Reporting Quality of COVID-19 Living Systematic Review: A Cross-Sectional Study, BMC Medical Research Methodology
Metzendorf, Weibel, Reis, Mcdonald, Pragmatic and Open Science-Based Solution to a Current Problem in the Reporting of Living Systematic Reviews, BMJ Evidence-Based Medicine
Mikolajewska, Fischer, Piechotta, Colchicine for the Treatment of COVID-19, Cochrane Database of Systematic Reviews
Millard, Synnot, Elliott, Green, Mcdonald et al., Feasibility and Acceptability of Living Systematic Reviews: Results From a Mixed-Methods Evaluation, Systematic Reviews
Nyirenda, Sofroniou, Toews, Fluvoxamine for the Treatment of COVID-19, Cochrane Database of Systematic Reviews
O'byrne, Webster, Mackeith, Philpott, Hopkins et al., Interventions for the Treatment of Persistent Post-COVID-19 Olfactory Dysfunction, Cochrane Database of Systematic Reviews
Popp, Stegemann, Metzendorf, Ivermectin for preventing and Treating COVID-19, Cochrane Database of Systematic Reviews
Popp, Stegemann, Riemer, Antibiotics for the Treatment of COVID-19, Cochrane Database of Systematic Reviews
Reis, Metzendorf, Kuehn, Nirmatrelvir Combined With Ritonavir for Preventing and Treating COVID-19, Cochrane Database of Systematic Reviews
Salameh, Leeflang, Hooft, Thoracic Imaging Tests for the Diagnosis of COVID-19, Cochrane Database of Systematic Reviews
Shojania, Sampson, Ansari, Ji, Doucette et al., How Quickly Do Systematic Reviews go Out of Date? A Survival Analysis, Annals of Internal Medicine
Stegeman, Ochodo, Guleid, Routine Laboratory Testing to Determine if a Patient has COVID-19, Cochrane Database of Systematic Reviews
Stroehlein, Wallqvist, Iannizzi, Vitamin D Supplementation for the Treatment of COVID-19: A Living Systematic Review, Cochrane Database of Systematic Reviews
Struyf, Deeks, Dinnes, Signs and Symptoms to Determine if a Patient Presenting in Primary Care or Hospital Outpatient Settings has COVID-19 Disease, Cochrane Database of Systematic Reviews
Tendal, Vogel, Mcdonald, Weekly Updates of National Living Evidence-Based Guidelines: Methods for the Australian Living Guidelines for Care of People With COVID-19, Journal of Clinical Epidemiology
Tong, Sainsbury, Craig, Consolidated Criteria for Reporting Qualitative Research (Coreq): A 32-Item Checklist for Interviews and Focus Groups, International Journal for Quality in Health Care
Valk, Piechotta, Chai, Convalescent Plasma or Hyperimmune Immunoglobulin for People With COVID-19: A Rapid Review, Cochrane Database of Systematic Reviews
Wagner, Griesel, Mikolajewska, Systemic Corticosteroids for the Treatment of COVID-19, Cochrane Database of Systematic Reviews
Wagner, Hirsch, Siemens, Kapp, Iannizzi, Experience Report of two Living Systematic Cochrane Reviews on COVID-19, Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen
Webster, O'byrne, Mackeith, Philpott, Hopkins et al., Interventions for the Prevention of Persistent Post-COVID-19 Olfactory Dysfunction, Cochrane Database of Systematic Reviews
Zheng, Xu, Gao, Past, Present and Future of Living Systematic Review: A Bibliometrics Analysis, BMJ Global Health
DOI record:
{
"DOI": "10.1002/cesm.70024",
"ISSN": [
"2832-9023",
"2832-9023"
],
"URL": "http://dx.doi.org/10.1002/cesm.70024",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Living systematic reviews (LSRs) should provide up‐to‐date evidence for priority questions where the evidence may be uncertain and fast‐moving. LSRs featured prominently during COVID‐19 and formed part of Cochrane's response to the pandemic. We conducted a mixed‐methods study to describe the characteristics of Cochrane's COVID‐19 living reviews, determine the currency of the included evidence, and evaluate authors' experiences in conducting and publishing these reviews.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We identified living reviews of COVID‐19 from the <jats:italic>Cochrane Database of Systematic Reviews</jats:italic> and extracted data on the number of versions published and publication timelines. We assessed the currency of evidence by comparing studies included in the reviews against a comprehensive list of studies maintained for the Australian living guidelines for COVID‐19. The qualitative component involved semi‐structured interviews with review authors to identify the barriers and enablers to conducting, reporting and publishing living reviews.</jats:p></jats:sec><jats:sec><jats:title>Findings</jats:title><jats:p>Cochrane published 25 COVID‐19 living systematic reviews. Half of these reviews had not been updated when assessed in June 2023 and only four had been updated more than once. A total of 118 studies were included in the living reviews. We estimated that an additional 119 studies were available and potentially relevant for inclusion. Interviews with six authors indicated that publication timelines were reduced by editorial delays, loss of funding, waning commitment, and the burden of screening search results. An inability to communicate the living status of reviews in the Cochrane Library was a common frustration for many authors. Although authors felt the conclusions of their reviews were still current, only one living review communicated its updated status and made new evidence accessible after the review was published.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Maintaining and communicating the currency of Cochrane's COVID‐19 living systematic reviews was not feasible for many author teams because of author‐side, editorial and platform barriers.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/cesm.70024"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-05-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-03-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-03-28"
}
],
"author": [
{
"ORCID": "https://orcid.org/0009-0007-7254-3484",
"affiliation": [
{
"name": "Cochrane Australia, School of Public Health and Preventive Medicine Monash University Melbourne Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "De Silva",
"given": "Kevindu",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-7990-1623",
"affiliation": [
{
"name": "Cochrane Australia, School of Public Health and Preventive Medicine Monash University Melbourne Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "Turner",
"given": "Tari",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2832-5205",
"affiliation": [
{
"name": "Cochrane Australia, School of Public Health and Preventive Medicine Monash University Melbourne Victoria Australia"
}
],
"authenticated-orcid": false,
"family": "McDonald",
"given": "Steve",
"sequence": "additional"
}
],
"container-title": "Cochrane Evidence Synthesis and Methods",
"container-title-short": "Cochrane Evidence Synthesis and Methods",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2025,
3,
30
]
],
"date-time": "2025-03-30T20:19:36Z",
"timestamp": 1743365976000
},
"deposited": {
"date-parts": [
[
2025,
5,
27
]
],
"date-time": "2025-05-27T04:40:24Z",
"timestamp": 1748320824000
},
"indexed": {
"date-parts": [
[
2025,
5,
28
]
],
"date-time": "2025-05-28T04:03:03Z",
"timestamp": 1748404983267,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2025,
3,
28
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2025,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
28
]
],
"date-time": "2025-03-28T00:00:00Z",
"timestamp": 1743120000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cesm.70024",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2025,
3,
28
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
28
]
]
},
"published-print": {
"date-parts": [
[
2025,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.jclinepi.2021.05.022",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.7326/0003-4819-147-4-200708210-00179",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.1016/j.jclinepi.2017.08.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1371/journal.pmed.1001603",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"key": "e_1_2_11_6_1",
"unstructured": "Living systematic reviews Cochrane Community: Cochrane Community; 2023 [Available from:https://community.cochrane.org/review-production/production-resources/living-systematic-reviews."
},
{
"DOI": "10.1136/bmj-2024-079183",
"article-title": "Extension of the PRISMA 2020 Statement for Living Systematic Reviews (Prisma‐Lsr): Checklist and Explanation",
"author": "Akl E. A.",
"doi-asserted-by": "crossref",
"journal-title": "BMJ",
"key": "e_1_2_11_7_1",
"volume": "387",
"year": "2024"
},
{
"DOI": "10.1186/s12874-023-01980-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.2147/CLEP.S367339",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1186/s13643-023-02325-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1136/bmjgh-2022-009378",
"article-title": "Past, Present and Future of Living Systematic Review: A Bibliometrics Analysis",
"author": "Zheng Q.",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "BMJ Global Health",
"key": "e_1_2_11_11_1",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1093/intqhc/mzm042",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1016/j.jclinepi.2020.11.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"article-title": "Nirmatrelvir Combined With Ritonavir for Preventing and Treating COVID‐19",
"author": "Reis S.",
"issue": "9",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_14_1",
"volume": "11",
"year": "2022"
},
{
"article-title": "Convalescent Plasma or Hyperimmune Immunoglobulin for People With COVID‐19: A Rapid Review",
"author": "Valk S. J.",
"issue": "5",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_15_1",
"volume": "1",
"year": "2020"
},
{
"article-title": "Hyperimmune Immunoglobulin for People With COVID‐19",
"author": "Kimber C.",
"issue": "1",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_16_1",
"volume": "1",
"year": "2023"
},
{
"article-title": "Systemic Corticosteroids for the Treatment of COVID‐19",
"author": "Wagner C.",
"issue": "8",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_17_1",
"volume": "8",
"year": "2021"
},
{
"article-title": "Inhaled Corticosteroids for the Treatment of COVID‐19",
"author": "Griesel M.",
"issue": "3",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_18_1",
"volume": "3",
"year": "2022"
},
{
"article-title": "Interventions for the Treatment of Persistent Post‐COVID‐19 Olfactory Dysfunction",
"author": "O'Byrne L.",
"issue": "7",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_19_1",
"volume": "1",
"year": "2021"
},
{
"article-title": "Janus Kinase Inhibitors for the Treatment of COVID‐19",
"author": "Kramer A.",
"issue": "6",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_20_1",
"volume": "6",
"year": "2022"
},
{
"article-title": "Colchicine for the Treatment of COVID‐19",
"author": "Mikolajewska A.",
"issue": "10",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_21_1",
"volume": "10",
"year": "2021"
},
{
"article-title": "SARS‐CoV‐2‐Neutralising Monoclonal Antibodies for Treatment of COVID‐19",
"author": "Kreuzberger N.",
"issue": "9",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_22_1",
"volume": "1",
"year": "2021"
},
{
"article-title": "Remdesivir for the Treatment of COVID‐19",
"author": "Ansems K.",
"issue": "8",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_23_1",
"volume": "1",
"year": "2021"
},
{
"article-title": "Vitamin D Supplementation for the Treatment of COVID‐19: A Living Systematic Review",
"author": "Stroehlein J. K.",
"first-page": "481",
"issue": "5",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_24_1",
"volume": "17",
"year": "2021"
},
{
"article-title": "Interleukin‐6 Blocking Agents for Treating COVID‐19: A Living Systematic Review",
"author": "Ghosn L.",
"issue": "3",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_25_1",
"volume": "3",
"year": "2021"
},
{
"article-title": "Fluvoxamine for the Treatment of COVID‐19",
"author": "Nyirenda J. L. Z.",
"issue": "9",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_26_1",
"volume": "9",
"year": "2022"
},
{
"article-title": "Ivermectin for preventing and Treating COVID‐19",
"author": "Popp M.",
"issue": "7",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_27_1",
"volume": "6",
"year": "2021"
},
{
"article-title": "Antibiotics for the Treatment of COVID‐19",
"author": "Popp M.",
"issue": "10",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_28_1",
"volume": "10",
"year": "2021"
},
{
"article-title": "Interleukin‐1 Blocking Agents for Treating COVID‐19",
"author": "Davidson M.",
"issue": "1",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_29_1",
"volume": "1",
"year": "2022"
},
{
"article-title": "Efficacy and Safety of COVID‐19 Vaccines",
"author": "Graña C.",
"issue": "12",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_30_1",
"volume": "12",
"year": "2022"
},
{
"article-title": "Interventions for the Prevention of Persistent Post‐COVID‐19 Olfactory Dysfunction",
"author": "Webster K. E.",
"issue": "7",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_31_1",
"volume": "7",
"year": "2021"
},
{
"article-title": "SARS‐CoV‐2‐Neutralising Monoclonal Antibodies to Prevent COVID‐19",
"author": "Hirsch C.",
"issue": "6",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_32_1",
"volume": "9",
"year": "2022"
},
{
"article-title": "Thoracic Imaging Tests for the Diagnosis of COVID‐19",
"author": "Salameh J. P.",
"issue": "9",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_33_1",
"volume": "5",
"year": "2020"
},
{
"article-title": "Signs and Symptoms to Determine if a Patient Presenting in Primary Care or Hospital Outpatient Settings has COVID‐19 Disease",
"author": "Struyf T.",
"issue": "7",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_34_1",
"volume": "5",
"year": "2020"
},
{
"article-title": "Routine Laboratory Testing to Determine if a Patient has COVID‐19",
"author": "Stegeman I.",
"issue": "11",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_35_1",
"volume": "1",
"year": "2020"
},
{
"article-title": "Rapid, Point‐of‐Care Antigen and Molecular‐Based Tests for Diagnosis of SARS‐CoV‐2 Infection",
"author": "Dinnes J.",
"issue": "8",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_36_1",
"volume": "8",
"year": "2020"
},
{
"article-title": "Antibody Tests for Identification of Current and Past infection With SARS‐CoV‐2",
"author": "Deeks J. J.",
"issue": "6",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_37_1",
"volume": "11",
"year": "2020"
},
{
"article-title": "Risk of Thromboembolism in Patients With COVID‐19 who Are Using Hormonal Contraception",
"author": "Cohen M. A.",
"issue": "1",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_38_1",
"volume": "1",
"year": "2023"
},
{
"DOI": "10.1136/bmjebm-2022-112019",
"article-title": "Pragmatic and Open Science‐Based Solution to a Current Problem in the Reporting of Living Systematic Reviews",
"author": "Metzendorf M. I.",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "BMJ Evidence‐Based Medicine",
"key": "e_1_2_11_39_1",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.7326/L22-0272",
"article-title": "Update Alert 8: Masks for Prevention of Respiratory Virus Infections, Including SARS‐CoV‐2, in Health Care and Community Settings",
"author": "Chou R.",
"doi-asserted-by": "crossref",
"first-page": "W108",
"issue": "9",
"journal-title": "Annals of Internal Medicine",
"key": "e_1_2_11_40_1",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.7326/M23-0570",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
},
{
"DOI": "10.1016/j.zefq.2023.11.004",
"article-title": "Experience Report of two Living Systematic Cochrane Reviews on COVID‐19",
"author": "Wagner C.",
"doi-asserted-by": "crossref",
"first-page": "90",
"journal-title": "Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen",
"key": "e_1_2_11_42_1",
"volume": "184",
"year": "2024"
},
{
"key": "e_1_2_11_43_1",
"unstructured": "Updating Classification System: guide to applying to Cochrane Reviews. Cochrane Editorial and Publishing Policy Resource: Cochrane Editorial and Methods Department;2019.https://documentation.cochrane.org/display/EPPR/Guide+to+applying+to+Cochrane+Reviews?preview=/117380724/117380820/Cochrane_UCS-Guide.pdf."
},
{
"article-title": "Electronic Cigarettes for Smoking Cessation",
"author": "Lindson N.",
"issue": "1",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_44_1",
"volume": "1",
"year": "2024"
},
{
"DOI": "10.1016/j.jclinepi.2023.111231",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_45_1"
},
{
"DOI": "10.1016/j.jclinepi.2021.09.013",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_46_1"
},
{
"DOI": "10.1186/s13643-019-1248-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_47_1"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.1002/CESM.70024/v1/review1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1002/CESM.70024/v1/decision1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1002/CESM.70024/v1/review2",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1002/CESM.70024/v2/response1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1002/CESM.70024/v3/decision1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1002/CESM.70024/v2/decision1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1002/CESM.70024/v3/response1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/cesm.70024"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Cochrane's COVID‐19 Living Systematic Reviews: A Mixed‐Methods Study of Their Conduct, Reporting and Currency",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "3"
}