The Efficacy of a Plant Based Formulation in the Symptomatic Management of Mild COVID-19 Cases: A Double Blind, Randomized Controlled Trial
et al., Archives of Clinical and Medical Case Reports, 7:1, CTRI/2021/08/036025, Jan 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 50 patients in India testing NAOQ19 which includes curcumin along with many other ingredients, showing improved recovery and viral clearance. SOC included vitamin C and zinc.
NAOQ19 is a plant based formulation with 19 ingredients from 13 herbs: Withania somnifera powder and extract, Aegle marmelos, Glycyrrhiza glabra powder and extract, Pluchea lanceolata, Adhatoda vasica powder and extract, Piper longum, Curcuma longa, Cissampelos pareira, Phyllanthus fraternus powder and extract, Andrographis paniculata powder and extract, Alstonia scholaris, Ocimum sanctum Tinospora cordifolia) powder and extract.
This study is excluded in meta-analysis:
many combined treatments which may significantly contribute to the effect seen.
|
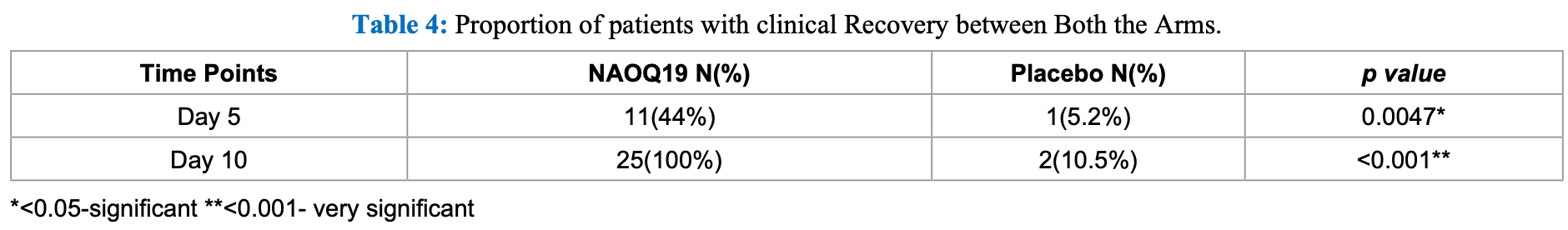
risk of no recovery, 97.5% lower, RR 0.02, p < 0.001, treatment 0 of 25 (0.0%), control 17 of 19 (89.5%), NNT 1.1, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 10.
|
|
risk of no recovery, 40.9% lower, RR 0.59, p = 0.006, treatment 14 of 25 (56.0%), control 18 of 19 (94.7%), NNT 2.6, day 5.
|
|
risk of no viral clearance, 76.0% lower, RR 0.24, p < 0.001, treatment 6 of 25 (24.0%), control 19 of 19 (100.0%), NNT 1.3, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Das et al., 18 Jan 2023, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, 7 authors, study period 1 September, 2021 - 25 September, 2021, trial CTRI/2021/08/036025.
The Efficacy of a Plant Based Formulation in the Symptomatic Management of Mild COVID-19 Cases: A Double Blind, Randomized Controlled Trial
Archives of Clinical and Medical Case Reports, doi:10.26502/acmcr.96550565
Background: The COVID-19 pandemic has infected millions of people globally. There is a need for integrated modern and traditional systems of medicine to work together to find effective solutions quickly. Methods: NAOQ19 is a plant based formulation of 13 herbs. 50 COVID-19 patients were enrolled in the study. RT-PCR analysis was done on day 1, day 5 and day 10. Clinical symptoms were noted daily. Result: NAOQ19 arm showed a higher population with RT-PCR negative compared to the placebo arm on Day 5 (76% vs 0%) On Day 5, NAOQ19 arm showed a complete recovery from few symptoms while by Day 10, they showed complete recovery from all symptoms, unlike the placebo arm which showed only 10.5% of the population with clinical recovery on day 10.
Conclusion: NAOQ19 facilitated a faster recovery from all clinical features of COVID-19, when compared to the placebo group. No side effects were observed during the entire study duration.
Author Contributions Conceptualization: Pradip Kumar
Conflict of Interest The test resources were provided by Sri Sri Tattva, / Sriveda Sattva Pvt Ltd, India. Dr Ravi Reddy is the chief scientific officer of Sriveda Sattva Pvt. Ltd., In addition Dr. Hari Venkatesh is the Research and Development head at Sriveda Sattva Pvt. Ltd. Besides providing the Intervention materials, Sriveda Sattva Pvt Ltd. was not involved in any aspect of this study. All the other authors have no conflicts of interest to declare.
Ethical Approval The study was approved by the Institutional Ethics Committee, Sri Sri University (IEC Number-SSCASRH/IEC/001/21) and registered with CTRI No. CTRI/2021/08/036025.
References
Armanini, Fiore, Bielenberg, Coronavirus-19: possible therapeutic implications of spironolactone and dry extract of Glycyrrhiza glabra L.(Licorice), Front. Pharmacol
Atkeson, Kopecky, Zha, Behavior and the Transmission of COVID-19, InAEA Papers and Proceedings
Bchetnia, Girard, Duchaine, The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review of the current global status, J. Infect. Public Health
Borse, Joshi, Saggam, Ayurveda botanicals in COVID-19 management: An in silico multi-target approach, PLoS One
Chowdhury, Hossain, Kashem, Immune response in COVID-19: A review, J. Infect. Public Health
Cohen, Tulsi-Ocimum sanctum: A herb for all reasons, J. Ayurveda Integr. Med
Devpura, Tomar, Nathiya, Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients, Phytomedicine
Duraipandiyan, Al-Dhabi, Balachandran, Antimicrobial, antioxidant, and cytotoxic properties of vasicine acetate synthesized from vasicine isolated from Adhatoda vasica L, BioMed Res. Int
Fernández-De-Las-Peñas, Palacios-Ceña, Gómez-Mayordomo, Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification, Int. J. Environ. Res. Public Health
Fraser, Long term respiratory complications of covid-19, BMJ
Gangwar, Ghosh, Medicinal uses and Das SK, Arch Clin Med Case Rep
Gheware, Panda, Khanna, Adhatoda vasica rescues the hypoxia-dependent severe asthma symptoms and mitochondrial dysfunction, Am. J. Physiol. Lung Cell Mol. Physiol
Hewlings, Kalman, Curcumin: A Review of Its' Effects on Human Health, Foods
Hossain, Hoq, Therapeutic use of Adhatoda vasica, Asian J. Med. Biol. Res
Hossain, Urbi, Sule, Andrographis paniculata (Burm. f.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology, Sci. World J
Kamal, Omirah, Hussein, Assessment and characterisation of post-COVID-19 manifestations, Int. J. Clin. Pract
Kanchibhotla, Subramanian, Hv, To study the in-vivo efficacy and safety of AYUSH polyherbal formulation among COVID-19 infected Syrian gold hamsters
Kanchibhotla, Subramanian, Reddy, An In-vitro evaluation of a polyherbal formulation, against SARS-CoV-2
Kapadiya, Dave, Harisha, Pharmacognostical and pharmaceutical analysis of amrutadi vati in the management of tamaka shwasa wsr to bronchial asthma, Pharm. Glob
Karthick, Adithya, Hariharaprasath, Evaluation of mechanical behavior of banana fibre reinforced hybrid epoxy composites, Mater. Today: Proc
Kashyap, Dhasmana, Yallapu, Withania somnifera as a potential future drug molecule for COVID-19, Future drug discovery
Khanal, Chikhale, Dey, Withanolides from Withania somnifera as an immunity booster and their therapeutic options against COVID-19
Kumar, Naik, Karra, Experimental and clinical evidence of Andrographis paniculata (Roxb.) Wall. Ex Nees (Bhunimba)-a review, Int. j. pharm. biol. sci. arch
Li, Zhang, Lu, Characteristics of household transmission of COVID-19, Clin. Infect. Dis
Lopresti, Smith, Ashwagandha (Withania somnifera) for the treatment and enhancement of mental and physical conditions: A systematic review of human trials, J. Herb. Med
Maurya, Evaluation of Yashtimadhu (Glycyrrhiza glabra) active phytochemicals against novel coronavirus (SARS-CoV-2)
Maurya, Evaluation of Yashtimadhu (Glycyrrhiza glabra) active phytochemicals against novel coronavirus (SARS-CoV-2)
Patel, Tripathi, Sharma, Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review, J. Ethnopharmacol
Sanjib Kumar Das, Prasad Dash, Kumar Panda, Sadana, Reddy M Ravi et al., The Efficacy of a Plant Based Formulation in the Symptomatic Management of Mild COVID-19 Cases: a Double Blind, Randomized Controlled Trial, Archives of Clinical and Medical Case Reports
Shree, Mishra, Selvaraj, Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants-Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)-a molecular docking study, J. Biomol. Struct. Dyn
Suvvari, Kutikuppala, Tsagkaris, Post-COVID-19 complications: Multisystemic approach, J. Med. Virol
Tandon, Yadav, Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments, J. Ethnopharmacol
Thakar, Panara, Patel, Add-on Ayurveda treatment for early stage COVID-19: a single center retrospective cohort study from Gujarat, India, J. Evid.-Based Integr. Med
Tripathi, Singh, Sharma, Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor, J. Biomol. Struct. Dyn
