Effectiveness of Hydroxychloroquine in COVID-19 disease: A done and dusted situation?
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.07.056, Jul 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
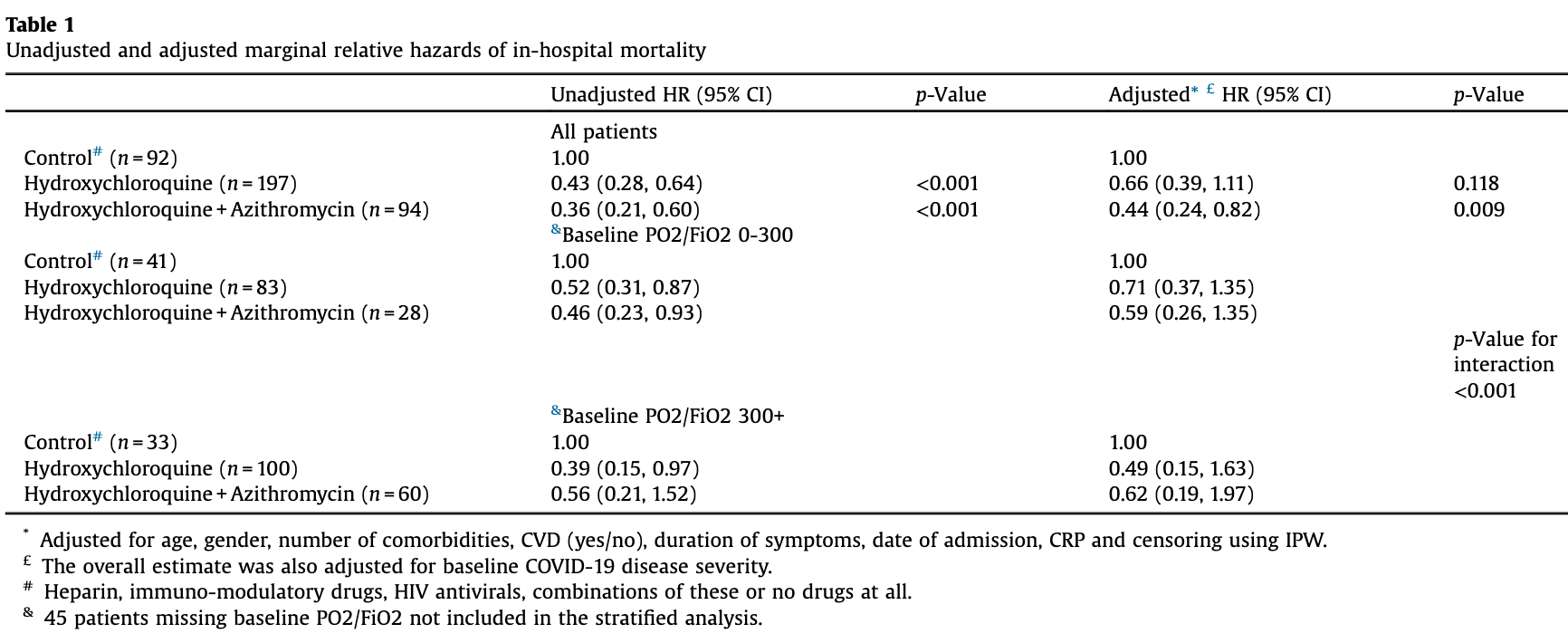
Retrospective 539 COVID-19 hospitalized patients in Milan, with treatment a median of 1 day after admission, showing lower mortality with HCQ and with HCQ+AZ, with statistical significance only for HCQ+AZ.
|
risk of death, 34.0% lower, HR 0.66, p = 0.12, treatment 53 of 197 (26.9%), control 47 of 92 (51.1%), NNT 4.1, adjusted per study.
|
|
HCQ+AZ, 56.0% lower, HR 0.44, p = 0.009, treatment 22 of 94 (23.4%), control 47 of 92 (51.1%), NNT 3.6, adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
D'Arminio Monforte et al., 29 Jul 2020, retrospective, Italy, peer-reviewed, 5 authors.
Effectiveness of hydroxychloroquine in COVID-19 disease: A done and dusted deal?
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.07.056
A total of 539 COVID-19 hospitalized patients were included in our cohort in Milan, from February 24 to May 17, 2020, of whom 174 died in hospital (day 14 probability of death: 29.5% -95%CI: 25.5-34.0). We divided a subset of our cohort into three groups who started treatment a median of 1 day after admission: those receiving hydroxychloroquine alone (N = 197), those receiving hydroxycholoroquine + azithromycin (N = 94), and those receiving neither (controls) (N = 92). Of the latter group, ten started HIV antivirals (boosted-lopinavir or -darunavir), one teicoplanin, twelve immunomodulatory drugs, or corticosteroids, 23 heparin and 46 remained untreated. The percent of death in the three groups was 27%, 23%, and 51%. Mechanical ventilation was used in 4.3% of hydroxychloroquine, 14.2% of hydroxychloroquine + azithromycin, and 26.1% of controls. Unweighted and weighted relative hazards of mortality are shown in Table 1 . After adjusting * Adjusted for age, gender, number of comorbidities, CVD (yes/no), duration of symptoms, date of admission, CRP and censoring using IPW. £ The overall estimate was also adjusted for baseline COVID-19 disease severity. # Heparin, immuno-modulatory drugs, HIV antivirals, combinations of these or no drugs at all. & 45 patients missing baseline PO2/FiO2 not included in the stratified analysis.
Declarations of interest None declared.
Ethical approval This analysis is part of the study approved by Ethic Committee Area 1, Milan Italy (2020/ST/049 and 2020/ST/049_BIS, 11/03/ 2020).
References
Arshad, Kigore, Chaudhry, Jacobsen, Wang et al., Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalised with COVID-19, Int J Infect Dis, doi:10.1016/j.ijid.2020.06.09
Devaux, Rolain, Colson, Raoult, New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, Int J Antimicrob Agents, doi:10.1016/j.antimicag.2020.105938
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomised clinical trial, Int J Antimicrob Agents, doi:10.1016/j.antimicag.2020.105949
Geleris, Sun, Platt, Zucker, Baldwin et al., Observational study on hydroxychloroquine in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2012410
Horby, Lim, Emberson, Mafham, Bell et al., Effect of desamethasone in hospitalized patients with CVID-19: preliminary report
Lee, Mackenzie, Mcdonald, Tong, An observational cohort study of hydroxychloroquine and azithromycin for COVID-19: (Can't get no) satisfaction, Int J Infect Dis, doi:10.1016/j.ijid.2020.06.095
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Tang, Cao, Han, Wang, Chen et al., Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomised, controlled trial, BMJ, doi:10.1136/bmj.m1849
DOI record:
{
"DOI": "10.1016/j.ijid.2020.07.056",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2020.07.056",
"alternative-id": [
"S1201971220306007"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effectiveness of hydroxychloroquine in COVID-19 disease: A done and dusted deal?"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2020.07.056"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/j.ijid.2020.06.099"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "d’Arminio Monforte",
"given": "Antonella",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tavelli",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bai",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marchetti",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cozzi-Lepri",
"given": "Alessandro",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ijidonline.com",
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.com.au",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
29
]
],
"date-time": "2020-07-29T16:31:01Z",
"timestamp": 1596040261000
},
"deposited": {
"date-parts": [
[
2020,
10,
24
]
],
"date-time": "2020-10-24T00:43:40Z",
"timestamp": 1603500220000
},
"indexed": {
"date-parts": [
[
2022,
4,
27
]
],
"date-time": "2022-04-27T06:48:55Z",
"timestamp": 1651042135584
},
"is-referenced-by-count": 7,
"issued": {
"date-parts": [
[
2020,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
27
]
],
"date-time": "2020-07-27T00:00:00Z",
"timestamp": 1595808000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971220306007?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971220306007?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "75-76",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.ijid.2020.06.099",
"article-title": "Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalised with COVID-19",
"author": "Arshad",
"doi-asserted-by": "crossref",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ijid.2020.07.056_bib0005",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105938",
"article-title": "New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?",
"author": "Devaux",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijid.2020.07.056_bib0010",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomised clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijid.2020.07.056_bib0015",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study on hydroxychloroquine in hospitalized patients with COVID-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"first-page": "2411",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2020.07.056_bib0020",
"volume": "382",
"year": "2020"
},
{
"author": "Horby",
"key": "10.1016/j.ijid.2020.07.056_bib0025",
"series-title": "Effect of desamethasone in hospitalized patients with CVID-19: preliminary report. COVID-19 SARS-CoV-2 preprints from medRxiv and bioRxiv",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.06.095",
"article-title": "An observational cohort study of hydroxychloroquine and azithromycin for COVID-19: (Can’t get no) satisfaction",
"author": "Lee",
"doi-asserted-by": "crossref",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ijid.2020.07.056_bib0030",
"year": "2020"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Cell Discov",
"key": "10.1016/j.ijid.2020.07.056_bib0035",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1849",
"article-title": "Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomised, controlled trial",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "m1849",
"journal-title": "BMJ",
"key": "10.1016/j.ijid.2020.07.056_bib0040",
"volume": "369",
"year": "2020"
},
{
"author": "World Health Organization",
"key": "10.1016/j.ijid.2020.07.056_bib0045",
"series-title": "“Solidarity” clinical trial for COVID-19 treatments – update on hydroxychloroquine",
"year": "2020"
}
],
"reference-count": 9,
"references-count": 9,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971220306007"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Effectiveness of hydroxychloroquine in COVID-19 disease: A done and dusted deal?",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "99"
}
