Disinfection Effect of Hexadecyl Pyridinium Chloride on SARS-CoV-2 in vitro
et al., Intervirology, doi:10.1159/000526241, Sep 2022
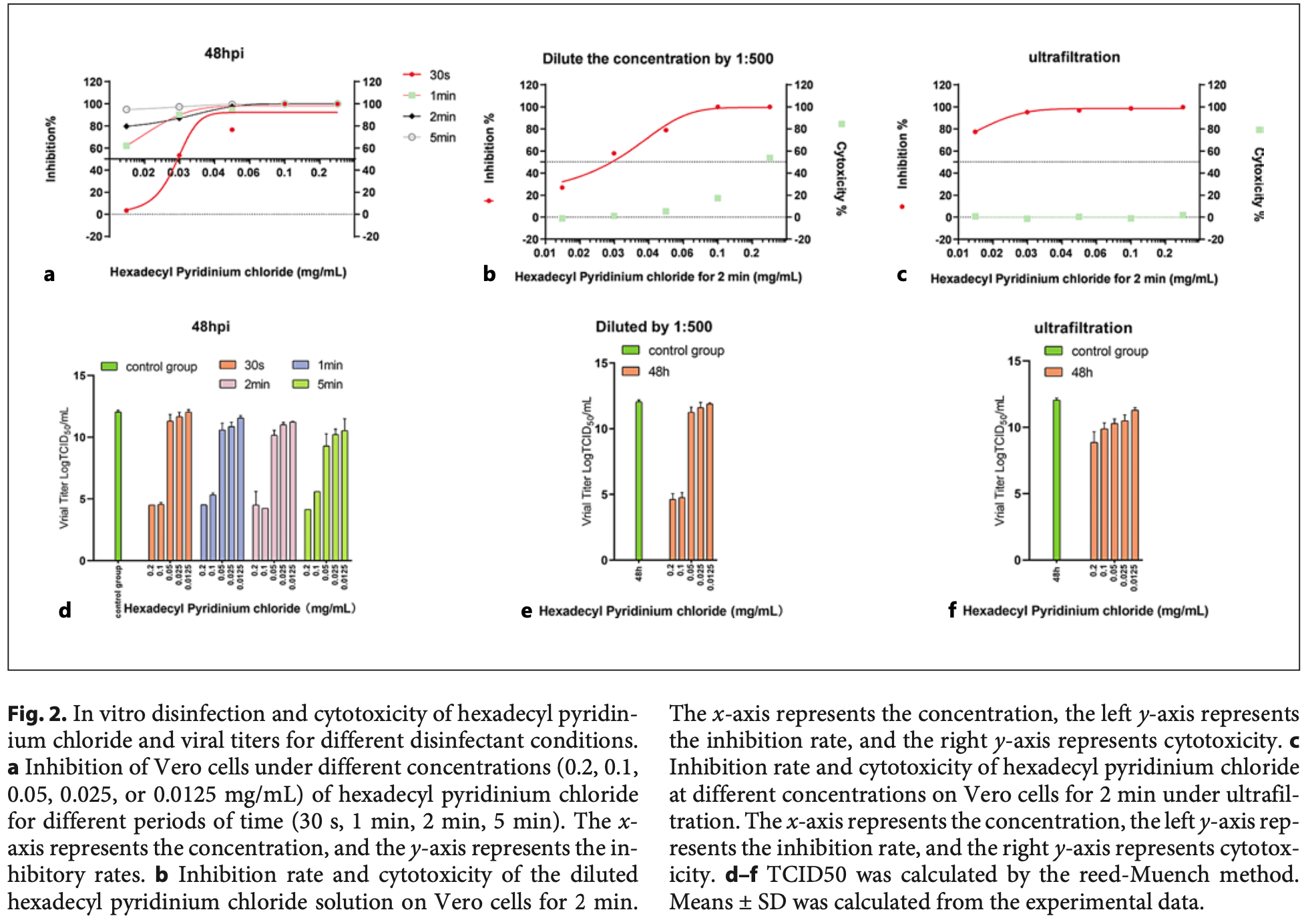
In vitro study showing that hexadecyl pyridinium chloride, an oral disinfectant, significantly inhibits SARS-CoV-2 in Vero cells at concentrations of 0.1 mg/mL or above after 2 minutes of exposure. The disinfection effect was time and concentration-dependent, with optimal results at 0.1 mg/mL for 2 minutes. Hexadecyl pyridinium chloride had no obvious cytotoxicity on Vero cells at concentrations of 0.0125-0.05 mg/mL. RT-PCR, immunofluorescence microscopy, and TCID50 testing confirmed the strong antiviral effect. Authors suggest hexadecyl pyridinium chloride could potentially reduce oral SARS-CoV-2 transmission.
Chen et al., 14 Sep 2022, China, peer-reviewed, 8 authors.
Contact: karger@karger.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Disinfection Effect of Hexadecyl Pyridinium Chloride on SARS-CoV-2 in vitro
Intervirology, doi:10.1159/000526241
The novel coronavirus (COVID-19 or 2019-nCoV) is a respiratory virus that can exist in the mouth and saliva of patients and spreads through aerosol dispersion. Therefore, stomatological hospitals and departments have become high-infection-risk environments. Accordingly, oral disinfectants that can effectively inactivate the virus have become a highly active area of research. Hexadecyl pyridinium chloride, povidone-iodine, and other common oral disinfectants are the natural primary choices for stomatological hospitals. Therefore, this study investigated the inhibitory effect of hexadecyl pyridinium chloride on severe acute respiratory syndrome coronavirus (SARS-CoV-2) in vitro. Vero cells infected with SARS-CoV-2 were used to determine the disinfection effect; the CCK-8 method was used to determine cytotoxicity, and viral load was determined by real-time PCR. The results showed that hexadecyl pyridinium chloride has no obvious cytotoxic effect on Vero cells in the concentration range of 0.0125-0.05 mg/mL. The in vitro experiments showed that hexadecyl pyridinium chloride significantly inhibits the virus at concentrations of 0.1 mg/mL or above at 2 min of action. Thus, the results provide experimental support for the use of hexadecyl pyridinium chloride in stomatological hospitals.
After dilution (1:500), 200 µL was used for immunofluorescence analysis. When the concentration is 0.1 mg/mL, the virus is com-pletely inhibited. The infected cells were fixed. Then, anti-spike RBD Rabbit PAb (1:1,000; Sino Biological) was used as the antibody and Alexa Fluor488 ® -conjugated Goat Anti-rabbit IgG (1:1,500; Abcam) was used as the second antibody. The nuclei were stained with DAPI. The scale is 100 µm.
Statement of Ethics This article does not contain any studies with human participants or animals.
Conflict of Interest Statement All the authors declare that they have no conflict of interest.
Author Contributions Ke-da Chen, Fei-ke Ma, and Qing-jing Wang contributed to conception, design, data acquisition, and analysis and drafted and critically revised the manuscript; Ying Wang, Xin-yi Zhuang, and Xu-ning Zhang assisted with data management and data analysis; Hai-yan Mao and Yan-jun Zhang conceived and designed the study. All the authors gave final approval and agree to be accountable for all aspects of the work. Disinfection Effect of Hexadecyl Pyridinium Chloride on SARS-CoV-2
References
Alvarez, Duarte, Corrales, Smith, González, Cetylpyridinium chloride blocks herpes simplex virus replication in gingival fibroblasts, Antiviral Res
Amato, Caggiano, Amato, Moccia, Capunzo et al., Infection control in dental practice during the CO-VID-19 pandemic, Int J Environ Res Public Health
Baker, Williams, Tropsha, Ekins, Repurposing quaternary ammonium compounds as potential treatments for CO-VID-19, Pharm Res
Carraturo, Giudice, Morelli, Cerullo, Libralato et al., Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces, Environ Pollut
Carrouel, Gonçalves, Conte, Campus, Fisher et al., Antiviral activity of reagents in mouth rinses against SARS-CoV-2, J Dent Res
Chakraborty, Sharma, Sharma, Bhattacharya, Lee, SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options, Eur Rev Med Pharmacol Sci
Chen, Ma, Wang, Zhuang, Zhang et al., Disinfection effect of hexadecyl pyridinium chloride on SARS-CoV-2 in vitro, Forthcoming
Corbett, Edwards, Leist, Abiona, Boyoglu-Barnum et al., SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness, Intervirology
Dowd, Covid-19: Cases of delta variant rise by 79%, but rate of growth slows, BMJ
Esakandari, Nabi-Afjadi, Fakkari-Afjadi, Farahmandian, Miresmaeili et al., A comprehensive review of COVID-19 characteristics, Biol Proced Online
Fini, What dentists need to know about COVID-19, Oral Oncol
Gao, Bao, Mao, Wang, Xu et al., Development of an inactivated vaccine candidate forSARS-CoV-2, Science
Islam, Al-Emran, Hasan, Anwar, Jahid et al., Emergence of European and North American mutant variants of SARS-CoV-2 in South-East Asia, Transbound Emerg Dis
Jiang, Li, Tang, Zhao, Zhang et al., Effect of different disinfectants on bacterial aerosol diversity in poultry houses, Front Microbiol
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents, Dermatology
Khokhar, Roy, Purohit, Goyal, Setia, Viricidal treatments for prevention of coronavirus infection, Pathog Glob Health
Mao, Auer, Buchalla, Hiller, Maisch et al., Cetylpyridinium chloride: mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance, Antimicrob Agents Chemother
Miranda, Damaceno, Faveri, Figueiredo, Soares et al., In vitro antimicrobial effect of cetylpyridinium chloride on complex multispecies subgingival biofilm, Braz Dent J
Rabenau, Kampf, Cinatl, Doerr, Efficacy of various disinfectants against SARS coronavirus, J Hosp Infect
Ramakrishnan, Determination of 50% endpoint titer using a simple formula, World J Virol
Rösing, Cavagni, Gaio, Muniz, Ranzan et al., Efficacy of two mouthwashes with cetylpyridinium chloride: a controlled randomized clinical trial, Braz Oral Res
Seneviratne, Balan, Ko, Udawatte, Lai et al., Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Seo, Seo, Cho, Ko, Kim et al., Cetylpyridinium chloride interaction with the hepatitis B virus core protein inhibits capsid assembly, Virus Res
Vergara-Buenaventura, Castro-Ruiz, Use of mouthwashes against COVID-19 in dentistry, Br J Oral Maxillofac Surg
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell
Yang, Tian, Liu, Strategies for vaccine development of COVID-19, Sheng Wu Gong Cheng Xue Bao
Yang, Yu, Xu, Shu, Xia et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med
Yao, Lu, Chen, Xu, Chen et al., Patient-derived SARS-CoV-2 mutations impact viral replication dynamics and infectivity in vitro and with clinical implications in vivo, Cell Discov
Yao, Song, Chen, Wu, Xu et al., Molecular architecture of the SARS-CoV-2 virus, Cell
Younes, Al-Sadeq, Al-Jighefee, Younes, Daas, Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2, Viruses
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
DOI record:
{
"DOI": "10.1159/000526241",
"ISSN": [
"0300-5526",
"1423-0100"
],
"URL": "http://dx.doi.org/10.1159/000526241",
"abstract": "<jats:p>The novel coronavirus (COVID-19 or 2019-nCoV) is a respiratory virus that can exist in the mouth and saliva of patients and spreads through aerosol dispersion. Therefore, stomatological hospitals and departments have become high-infection-risk environments. Accordingly, oral disinfectants that can effectively inactivate the virus have become a highly active area of research. Hexadecyl pyridinium chloride, povidone-iodine, and other common oral disinfectants are the natural primary choices for stomatological hospitals. Therefore, this study investigated the inhibitory effect of hexadecyl pyridinium chloride on severe acute respiratory syndrome coronavirus (SARS-CoV-2) in vitro. Vero cells infected with SARS-CoV-2 were used to determine the disinfection effect; the CCK-8 method was used to determine cytotoxicity, and viral load was determined by real-time PCR. The results showed that hexadecyl pyridinium chloride has no obvious cytotoxic effect on Vero cells in the concentration range of 0.0125–0.05 mg/mL. The in vitro experiments showed that hexadecyl pyridinium chloride significantly inhibits the virus at concentrations of 0.1 mg/mL or above at 2 min of action. Thus, the results provide experimental support for the use of hexadecyl pyridinium chloride in stomatological hospitals. </jats:p>",
"author": [
{
"affiliation": [],
"family": "Chen",
"given": "Ke-da",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ma",
"given": "Fei-ke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Qing-jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Ying",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9960-9243",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhuang",
"given": "Xin-yi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0605-9882",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Xu-ning",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mao",
"given": "Hai-yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Yan-jun",
"sequence": "additional"
}
],
"container-title": "Intervirology",
"container-title-short": "Intervirology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
14
]
],
"date-time": "2022-09-14T21:00:26Z",
"timestamp": 1663189226000
},
"deposited": {
"date-parts": [
[
2024,
5,
18
]
],
"date-time": "2024-05-18T07:49:15Z",
"timestamp": 1716018555000
},
"indexed": {
"date-parts": [
[
2024,
5,
19
]
],
"date-time": "2024-05-19T00:55:51Z",
"timestamp": 1716080151732
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
9,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12,
20
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
14
]
],
"date-time": "2022-09-14T00:00:00Z",
"timestamp": 1663113600000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
14
]
],
"date-time": "2022-09-14T00:00:00Z",
"timestamp": 1663113600000
}
}
],
"link": [
{
"URL": "https://karger.com/int/article-pdf/66/1/8/3962199/000526241.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://karger.com/int/article-pdf/66/1/8/3962199/000526241.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "127",
"original-title": [],
"page": "8-15",
"prefix": "10.1159",
"published": {
"date-parts": [
[
2022,
9,
14
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
14
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "S. Karger AG",
"reference": [
{
"DOI": "10.26355/eurrev_202004_20871",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1186/s12575-020-00128-2",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.13345/j.cjb.200094",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.3390/v12060582",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.jhin.2004.12.023",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1159/000089211",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1016/j.cell.2020.09.018",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1111/tbed.13748",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/j.oraloncology.2020.104741",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1177/0022034520967933",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.bjoms.2020.08.016",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.virusres.2019.01.004",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1590/0103-6440202002630",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1007/s11095-020-02842-8",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.5501/wjv.v5.i2.85",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1038/s41586-020-2622-0",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.envpol.2020.115010",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.3390/ijerph17134769",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1080/20477724.2020.1807177",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.antiviral.2020.104818",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1128/AAC.00576-20",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1038/s41421-020-00226-1",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.21203/rs.3.rs-458096/v2",
"doi-asserted-by": "publisher",
"key": "ref27"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://karger.com/INT/article/doi/10.1159/000526241"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Disinfection Effect of Hexadecyl Pyridinium Chloride on SARS-CoV-2 in vitro",
"type": "journal-article",
"volume": "66"
}