Hydrogen Peroxide as an Adjuvant Therapy for COVID-19: A Case Series of Patients and Caregivers in the Mexico City Metropolitan Area
et al., Evidence-Based Complementary and Alternative Medicine, doi:10.1155/2021/5592042, Jul 2021
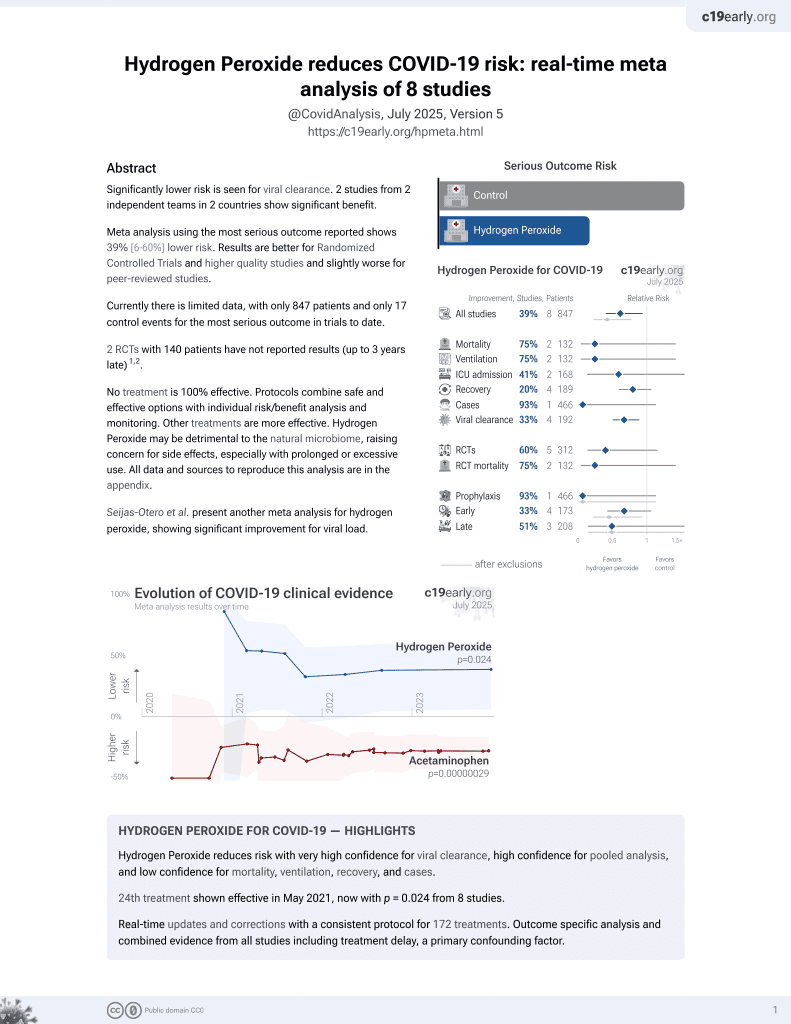
24th treatment shown to reduce risk in
May 2021, now with p = 0.024 from 8 studies.
Lower risk for viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Case series of 23 COVID-19 patients and 28 caregivers in Mexico using hydrogen peroxide treatment and prophylaxis. There was no transmission to caregivers. Patients mainly recovered well, reporting feeling “completely better” at 9.5 days on average. Two (9%) were hospitalized prior to joining the study, and one did not fully recover.
Cervantes Trejo et al., 3 Jul 2021, Randomized Controlled Trial, Mexico, peer-reviewed, mean age 39.0, 10 authors, study period 11 May, 2020 - 19 July, 2020.
Contact: arturo.cervantes@anahuac.mx.
Hydrogen Peroxide as an Adjuvant Therapy for COVID-19: A Case Series of Patients and Caregivers in the Mexico City Metropolitan Area
Evidence-Based Complementary and Alternative Medicine, doi:10.1155/2021/5592042
Knowledge of the antiseptic effects of hydrogen peroxide (H 2 O 2 ) dates back to the late 19th century, and its mechanisms of action has been amply described. Globally, many physicians have reported using H 2 O 2 successfully, in different modalities, against COVID-19. Given its anti-infective and oxygenating properties, hydrogen peroxide may offer prophylactic and therapeutic applications for responding to the COVID-19 pandemic. We report a consecutive case series of twenty-three COVID-19 patients (of 36 initially enrolled) who had been diagnosed by their primary care physician (mean age: 39, range: 8 months-70 years; 74% male) and twenty-eight caregivers in the Mexico City Metropolitan Area who received a complementary and alternative medicine (CAM) telemedicine treatment with H 2 O 2 taken by mouth (PO, at a concentration of 0.06%), oral rinse (mouthwash, 1.5%), and/ or nebulization (0.2%). We describe the treatment program and report the response of the COVID-19 patients and their caregivers. e patients mainly recovered well, reporting feeling "completely better" at 9.5 days on average. Two (9%) were hospitalized prior to joining the study, and one did not fully recover. Patients frequently reported nausea and sometimes dizziness or vomiting related to the oral treatment. None of the twenty-eight caregivers in close contact with the patients reported contracting COVID-19. Given its low cost and medical potential and considering its relative safety if used properly, we suggest that randomized controlled trials should be conducted. ese should include both SARS-CoV-2-positive and SARS-CoV-2negative participants, with single or combined modes of administration of H 2 O 2 , to study the benefits of this simple molecule and offer safe guidance regarding its use by health professionals.
Ethical Approval According to the Declaration of Helsinki, which literally states: Article 37 "When in the patient care the proven interventions there are no other known interventions, they have been ineffective, e doctor, after requesting expert advice, with the informed consent of the patient or of an authorized legal representative, can afford to use unproven interventions, if in their judgment, they give some hope of saving life, restoring health, or alleviating suffering. Such interventions should be further investigated to assess their safety and efficacy. In all cases, this new information must be recorded and, when appropriate, made available to the public." is research is based on the principles of personalistic Bioethics, given that the human person with their intrinsic dignity is considered as a core value. is was demonstrated by caring for the wellbeing of patients regardless of age, comorbidity, severity, or prognosis, seeking their improvement comprehensively. We have complied with benefitting the common good by offering curative or prophylactic treatment to patients, health personnel, caregivers, and relatives hoping to avoid contagion or decrease the severity of the disease. is study complies with the universal principles in ethics during research in human beings and the Declaration of Helsinki. By not having an effective treatment for COVID-19 and given the morbidity and mortality of the disease, the exploration of a known solution with low-risk..
References
Atwood, Charles, Farr and the purported scientific and medical rationale for intravenous hydrogen peroxide, Scientific Review of Alternative Medicine
Brownstein, Richard, Rowen, A novel approach to treating COVID-19 using nutritional and oxidative therapies, Science, Public Health Policy, and e Law
Caruso, Del Prete, Lazzarino, Capaldi, Grumetto, Might hydrogen peroxide reduce the hospitalization rate and complications of SARS-CoV-2 infection?, Infection Control and Hospital Epidemiology
Caruso, Del Prete, Lazzarino, Hydrogen peroxide and viral infections: a literature review with research hypothesis definition in relation to the current covid-19 pandemic, Medical Hypotheses
Cavalcante-Leão, De Araujo, Basso, Is there scientific evidence of the mouthwashes effectiveness in reducing viral load in Covid-19? A systematic review, Journal of Clinical and Experimental Dentistry
Cavanaugh, One-Minute Cure: e Secret to Healing Virtually All Diseases, ink Outside the
Douglass, Hydrogen Peroxide-Medical Miracle
Farr, Workbook on Free Radical Chemistry and Hydrogen Peroxide Metabolism. Including Protocol for the Intravenous Administration of Hydrogen Peroxide, IBOM Foundation
Farr, erapeutic Use of Intravenous Hydrogen Peroxide
Fowler, Moeller, Roa, Projected impact of COVID-19 mitigation strategies on hospital services in the Mexico City Metropolitan area, PLoS One
Gansky, UCSF COVID-19 trial: effect of antiseptic mouthwash/gargling solutions and pre-procedural Rinse on SARS-CoV-2 Load (COVID-19)
Gold, Hydrogen_Peroxide H2O2 therapy
González, López, Super-oxidized solution nebulization for the symptomatic treatment of airway infections including COVID-19 cases report, International Journal of Innovative Research in Medical Science
Gottsauner, Michaelides, Schmidt, A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2, Clinical Oral Investigations
Goyal, Chander, Yezli, Otter, Evaluating the virucidal efficacy of hydrogen peroxide vapour, Journal of Hospital Infection
Halliwell, Clement, Long, Hydrogen peroxide in the human body, FEBS Letters
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19, Nature Reviews Microbiology
Kampf, Todt, Pfaender, Steinmann, Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents, Journal of Hospital Infection
Khan, Kazmi, Iqbal, Iqbal, Ali et al., A quadruple blind, randomised controlled trial of gargling agents in reducing intraoral viral load among hospitalised COVID-19 patients: a structured summary of a study protocol for a randomised controlled trial, Trials
Love, Peroxide of hydrogen as a remedial agent: read before the St, Journal of the American Medical Association
Marshall, Cancro, Fischman, Hydrogen peroxide: a review of its use in dentistry, Journal of Periodontology
Matheson, Lehner, How does SARS-CoV-2 cause COVID-19?, Science
Meiller, Silva, Ferreira, Jabra-Rizk, Kelley et al., Efficacy of Listerine ® antiseptic in reducing viral contamination of saliva, Journal of Clinical Periodontology
Oliver, Murphy, Influenzal pneumonia: the intravenous injection of hydrogen peroxide, Lancet
Ortega, Rech, Ferreira Costa, Pérez, Sayans et al., Is 0.5% hydrogen peroxide effective against SARS-CoV-2?
Pasten, Lineamiento para la atención de pacientes por COVID-19: CVOED-centro virtual de operaciones en emergencias y desastres
Vergara-Buenaventura, Castro-Ruiz, Use of mouthwashes against COVID-19 in dentistry, British Journal of Oral and Maxillofacial Surgery
William, Mauroy, Farnir, Iry, Virucidal efficacy of a hydrogen peroxide nebulization against murine norovirus and feline calicivirus, two surrogates of human norovirus, Food and Environmental Virology
Williams, Hydrogen Peroxide Protocol
Yoon, Yoon, Song, Clinical significance of a high SARS-CoV-2 viral load in the saliva, Journal of Korean Medical Science
DOI record:
{
"DOI": "10.1155/2021/5592042",
"ISSN": [
"1741-4288",
"1741-427X"
],
"URL": "http://dx.doi.org/10.1155/2021/5592042",
"abstract": "<jats:p>Knowledge of the antiseptic effects of hydrogen peroxide (H2O2) dates back to the late 19th century, and its mechanisms of action has been amply described. Globally, many physicians have reported using H2O2 successfully, in different modalities, against COVID-19. Given its anti-infective and oxygenating properties, hydrogen peroxide may offer prophylactic and therapeutic applications for responding to the COVID-19 pandemic. We report a consecutive case series of twenty-three COVID-19 patients (of 36 initially enrolled) who had been diagnosed by their primary care physician (mean age: 39, range: 8 months–70 years; 74% male) and twenty-eight caregivers in the Mexico City Metropolitan Area who received a complementary and alternative medicine (CAM) telemedicine treatment with H2O2 taken by mouth (PO, at a concentration of 0.06%), oral rinse (mouthwash, 1.5%), and/or nebulization (0.2%). We describe the treatment program and report the response of the COVID-19 patients and their caregivers. The patients mainly recovered well, reporting feeling “completely better” at 9.5 days on average. Two (9%) were hospitalized prior to joining the study, and one did not fully recover. Patients frequently reported nausea and sometimes dizziness or vomiting related to the oral treatment. None of the twenty-eight caregivers in close contact with the patients reported contracting COVID-19. Given its low cost and medical potential and considering its relative safety if used properly, we suggest that randomized controlled trials should be conducted. These should include both SARS-CoV-2-positive and SARS-CoV-2-negative participants, with single or combined modes of administration of H2O2, to study the benefits of this simple molecule and offer safe guidance regarding its use by health professionals.</jats:p>",
"alternative-id": [
"5592042",
"5592042"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7107-9217",
"affiliation": [
{
"name": "Carlos Peralta Professor and Chair of Public Health, Anahuac Institute of Public Health, Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"authenticated-orcid": true,
"family": "Cervantes Trejo",
"given": "Arturo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Carlos Peralta Chair of Public Health, Anahuac Institute of Public Health, Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Castañeda",
"given": "Isaac D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bursary Scholar of Medicine, Anahuac Institute of Public Health, Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Rodríguez",
"given": "Alejandra Cortés",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bursary Scholar of Medicine, Anahuac Institute of Public Health, Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Andrade Carmona",
"given": "Victor R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Bioethics and Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Mercado",
"given": "M. del Pilar Calva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bursary Scholar of Medicine, Anahuac Institute of Public Health, Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Vale",
"given": "Liliana Salgado",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine, Universidad de Monterrey, San Pedro Garza García, Mexico"
}
],
"family": "Cruz",
"given": "Montserrat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Barrero Castillero",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bursary Scholar of Medicine, Anahuac Institute of Public Health, Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Consuelo",
"given": "Lucero Chavez",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fellow of the American College of Surgeons (FACS), Medical Director of Hospital Mac Periferico Sur, Mexico City, Coyoacan 04700, Mexico"
},
{
"name": "Faculty of Health Sciences, Anahuac University Mexico, Naucalpan de Juárez 52786, Mexico"
}
],
"family": "Di Silvio",
"given": "Mauricio",
"sequence": "additional"
}
],
"container-title": "Evidence-Based Complementary and Alternative Medicine",
"container-title-short": "Evidence-Based Complementary and Alternative Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
3
]
],
"date-time": "2021-07-03T19:35:08Z",
"timestamp": 1625340908000
},
"deposited": {
"date-parts": [
[
2021,
7,
17
]
],
"date-time": "2021-07-17T05:57:25Z",
"timestamp": 1626501445000
},
"editor": [
{
"affiliation": [],
"family": "Solano",
"given": "Francisco",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
1
]
],
"date-time": "2023-04-01T14:53:57Z",
"timestamp": 1680360837221
},
"is-referenced-by-count": 7,
"issued": {
"date-parts": [
[
2021,
7,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
3
]
],
"date-time": "2021-07-03T00:00:00Z",
"timestamp": 1625270400000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/ecam/2021/5592042.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ecam/2021/5592042.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ecam/2021/5592042.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "98",
"original-title": [],
"page": "1-12",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2021,
7,
3
]
]
},
"published-print": {
"date-parts": [
[
2021,
7,
3
]
]
},
"publisher": "Hindawi Limited",
"reference": [
{
"DOI": "10.1001/jama.1888.02400350010002a",
"doi-asserted-by": "publisher",
"key": "1"
},
{
"DOI": "10.1016/s0140-6736(01)11118-9",
"doi-asserted-by": "publisher",
"key": "2"
},
{
"article-title": "Farr and the purported scientific and medical rationale for intravenous hydrogen peroxide",
"author": "K. Atwood",
"first-page": "11",
"issue": "1",
"journal-title": "Scientific Review of Alternative Medicine",
"key": "3",
"volume": "11",
"year": "2007"
},
{
"DOI": "10.1016/s0014-5793(00)02197-9",
"doi-asserted-by": "publisher",
"key": "4"
},
{
"author": "C. H. Farr",
"key": "5",
"volume-title": "The Therapeutic Use of Intravenous Hydrogen Peroxide",
"year": "1986"
},
{
"author": "C. H. Farr",
"key": "6",
"volume-title": "Workbook on Free Radical Chemistry and Hydrogen Peroxide Metabolism. Including Protocol for the Intravenous Administration of Hydrogen Peroxide",
"year": "1996"
},
{
"DOI": "10.1016/j.bjoms.2020.08.016",
"doi-asserted-by": "publisher",
"key": "7"
},
{
"DOI": "10.1902/jop.1995.66.9.786",
"doi-asserted-by": "publisher",
"key": "8"
},
{
"article-title": "A novel approach to treating COVID-19 using nutritional and oxidative therapies",
"author": "D. Brownstein",
"first-page": "4",
"journal-title": "Science, Public Health Policy, and The Law",
"key": "9",
"volume": "2",
"year": "2020"
},
{
"article-title": "Coronavirus (COVID-19) update: FDA issues second emergency use authorization to decontaminate N95 respirators",
"author": "Office of the Commissioner",
"key": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.jhin.2014.02.003",
"doi-asserted-by": "publisher",
"key": "11"
},
{
"DOI": "10.1111/odi.13503",
"doi-asserted-by": "publisher",
"key": "12"
},
{
"DOI": "10.1016/j.jhin.2020.01.022",
"doi-asserted-by": "publisher",
"key": "13"
},
{
"DOI": "10.1017/ice.2020.170",
"doi-asserted-by": "publisher",
"key": "14"
},
{
"DOI": "10.1016/j.mehy.2020.109910",
"doi-asserted-by": "publisher",
"key": "15"
},
{
"article-title": "UCSF COVID-19 trial: effect of antiseptic mouthwash/gargling solutions and pre-procedural Rinse on SARS-CoV-2 Load (COVID-19)",
"author": "S. A. Gansky",
"key": "16",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04634-2",
"doi-asserted-by": "publisher",
"key": "17"
},
{
"DOI": "10.1371/journal.pone.0241954",
"doi-asserted-by": "publisher",
"key": "18"
},
{
"key": "19"
},
{
"DOI": "10.1038/s41579-020-00459-7",
"doi-asserted-by": "publisher",
"key": "20"
},
{
"DOI": "10.1126/science.abc6156",
"doi-asserted-by": "publisher",
"key": "21"
},
{
"author": "M. Cavanaugh",
"edition": "1st",
"key": "22",
"volume-title": "The One-Minute Cure: The Secret to Healing Virtually All Diseases",
"year": "2008"
},
{
"DOI": "10.23958/ijirms/vol05-i08/922",
"doi-asserted-by": "publisher",
"key": "23"
},
{
"author": "W. C. Douglass",
"key": "24",
"volume-title": "Hydrogen Peroxide-Medical Miracle",
"year": "2003"
},
{
"article-title": "Hydrogen_Peroxide H2O2 therapy",
"author": "C. M. Gold",
"key": "25",
"year": "2015"
},
{
"key": "26"
},
{
"DOI": "10.3346/jkms.2020.35.e195",
"doi-asserted-by": "publisher",
"key": "27"
},
{
"DOI": "10.1111/j.1600-051x.2005.00673.x",
"doi-asserted-by": "publisher",
"key": "28"
},
{
"DOI": "10.4317/jced.57406",
"doi-asserted-by": "publisher",
"key": "29"
},
{
"DOI": "10.1007/s00784-020-03549-1",
"doi-asserted-by": "publisher",
"key": "30"
},
{
"article-title": "Lineamiento para la atención de pacientes por COVID-19: CVOED-centro virtual de operaciones en emergencias y desastres",
"author": "J. C. M. Pasten",
"key": "31",
"year": "2020"
},
{
"DOI": "10.1007/s12560-016-9253-5",
"doi-asserted-by": "publisher",
"key": "32"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/ecam/2021/5592042/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Complementary and alternative medicine"
],
"subtitle": [],
"title": "Hydrogen Peroxide as an Adjuvant Therapy for COVID-19: A Case Series of Patients and Caregivers in the Mexico City Metropolitan Area",
"type": "journal-article",
"volume": "2021"
}