Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial
et al., Scientific Reports, doi:10.1038/s41598-022-14601-3, ACTRN12620001371987, Jun 2022
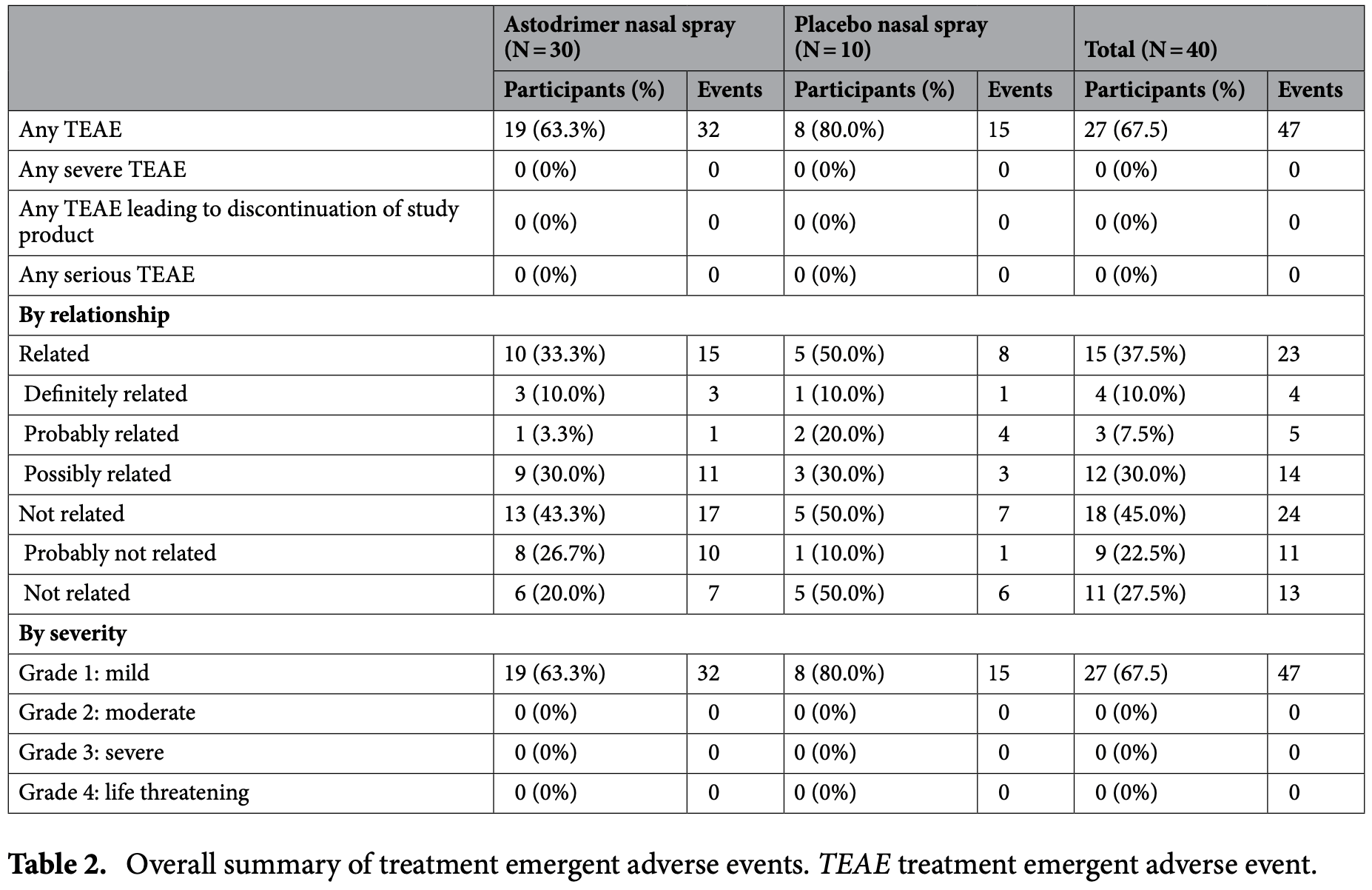
RCT 40 healthy volunteers showing astodrimer sodium 1% nasal spray was well tolerated with no systemic absorption detected. Treatment emergent adverse events occurred in a greater proportion of participants receiving placebo than astodrimer. All adverse events were mild.
Castellarnau et al., 17 Jun 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Australia, peer-reviewed, 8 authors, study period 6 January, 2021 - 29 March, 2021, trial ACTRN12620001371987.
Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial
Scientific Reports, doi:10.1038/s41598-022-14601-3
Astodrimer sodium is a dendrimer molecule with antiviral and virucidal activity against SARS-CoV-2 and other respiratory viruses in vitro, and has previously been shown to be safe and well tolerated, and not systemically absorbed, when applied to the vaginal mucosa. To investigate its potential utility as a topical antiviral, astodrimer sodium has been reformulated for application to the nasal mucosa to help reduce viral load before or after exposure to respiratory infection. The current investigation assessed the safety, tolerability and absorption of astodrimer sodium 1% antiviral nasal spray. This was a singlecentre, double-blinded, randomized, placebo-controlled, exploratory clinical investigation. Forty healthy volunteers aged 18 to 65 years with no clinically significant nasal cavity examination findings were randomized 3:1 to astodrimer sodium nasal spray (N = 30) or placebo (N = 10) at an Australian clinical trials facility. An initial cohort of participants (N = 12 astodrimer, N = 4 placebo) received a single application (one spray per nostril) to assess any acute effects, followed by a washout period, before self-administering the spray four times daily for 14 days to represent an intensive application schedule. Extent of absorption of astodrimer sodium via the nasal mucosa was also assessed in this cohort. A second cohort of participants (N = 18 astodrimer, N = 6 placebo) self-administered the spray four times daily for 14 days. The primary endpoint was safety, measured by frequency and severity of treatment emergent adverse events (TEAEs), including clinically significant nasal cavity examination findings, in the safety population (all participants randomized who administered any spray). Participants were randomized between 6 January 2021 and 29 March 2021. TEAEs occurred in 8/10 (80%) participants in the placebo arm and 19/30 (63.3%) participants in the astodrimer sodium arm; all were of mild intensity. TEAEs considered potentially related to study product occurred in 5/10 (50%) participants receiving placebo and 10/30 (33.3%) of participants receiving astodrimer sodium. No participants experienced serious AEs, or TEAEs leading to withdrawal from the study. No systemic absorption of astodrimer sodium via the nasal mucosa was detected. Astodrimer sodium nasal spray was well tolerated and is a promising innovation warranting further investigation for nasal administration to potentially reduce infection and spread of community acquired respiratory virus infections. Trial Registration: ACTRN12620001371987, first registered 22-12-2020 (Australia New Zealand Clinical Trials Registry, https:// anzctr. org. au/). Epidemic and pandemic respiratory viral infections have caused significant public health problems worldwide. Recent examples of pandemic viruses include the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and influenza A virus (H1N1/pdm/09). Each of these pandemics has been associated with the emergence of..
Ethics approval and consent to participate. The investigation was approved by the Bellberry Human Research Ethics Committee (EC00469; SA, Australia) on 21 December 2020. All participants provided written informed consent prior to screening procedures.
Author contributions
Competing interests The authors JRAP, GPH and AS are paid employees of Starpharma Pty Ltd. The authors AC, CAL, GRK, PB and PMc are paid consultants to Starpharma Pty Ltd. JRAP, GPH, AS, AC and CAL are shareholders of Starpharma Holdings Ltd.
Additional information Correspondence and requests for materials should be addressed to J.R.A.P. Reprints and permissions information is available at www.nature.com/reprints. Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abu-Zaid, Astodrimer gel for treatment of bacterial vaginosis: A systematic review and meta-analysis of randomized controlled trials, Int. J. Clin. Pract, doi:10.1111/ijcp.14165
Bernstein, Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes, Antimicrob. Agents Chemother, doi:10.1128/AAC.47.12.3784-3788.2003
Chavoustie, Two phase 3, double-blind, placebo-controlled studies of the efficacy and safety of Astodrimer 1% Gel for the treatment of bacterial vaginosis, Eur. J. Obstet. Gynecol. Rep. Biol, doi:10.1016/j.ejogrb.2019.11.032
Eccles, Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial, Respir. Res, doi:10.1186/s12931-015-0281-8
Gallay, Effects of astodrimer sodium against SARS-CoV-2 variants (α, β, γ, δ, κ) in vitro
Gong, Evaluation of Dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses, Antiviral Res, doi:10.1016/j.antiviral.2005.08.004
Graf, Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis, Int. J. Gen. Med, doi:10.2147/IJGM.S167123
Jiang, SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques, AIDS Res. Hum. Retroviruses, doi:10.1089/aid.2005.21.207
Lewis, Is the coronavirus airborne? Experts can't agree, Nature, doi:10.1038/d41586-020-00974-w
Ludwig, Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial, Respir. Res, doi:10.1186/1465-9921-14-124
Mccarthy, Dendrimers as drugs: Discovery and preclinical and clinical development of Dendrimer-based microbicides for HIV and STI, Mol. Pharm, doi:10.1021/mp050023q
Mccray, Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus, J. Virol, doi:10.1128/JVI.02012-06
Mcgowan, Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel®) in sexually active young women (MTN-004), AIDS, doi:10.1097/QAD.0b013e328346bd3e
O'loughlin, Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel®): A dose ranging phase 1 study, Sex Transm. Dis, doi:10.1097/OLQ.0b013e3181bc0aac
Ong, Epidemic and pandemic viral infection: Impact on tuberculosis and the lung: A consensus by the World Association for Infectious Diseases and Immunological Disorders (WAidid), Global Tuberculosis Network (GTN), and members of the European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycobacterial Infections (ESGMYC), Eur. Respir. J, doi:10.1183/13993003.01727-2020
Paull, Protective effects of astodrimer sodium 1% nasal spray formulation against SARS-CoV-2 nasal challenge in K18-hACE2 mice, Viruses, doi:10.3390/v13081656
Paull, Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2021.105089
Price, SPL7013 (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans, PLoS ONE, doi:10.1371/journal.pone.0024095
Ratcliffe, Nasal therapy-The missing link in optimising strategies to improve prevention and treatment of COVID-19, PLoS Pathog, doi:10.1371/journal.ppat.1010079
Schwebke, A phase 3, randomized, controlled trial of Astodrimer 1% Gel for preventing recurrent bacterial vaginosis, Eur. J. Obstet. Gynecol. Rep. Biol. X, doi:10.1016/j.eurox.2021.100121
Tang, Aerosol transmission of SARS-CoV-2? Evidence, prevention and control, Environ. Int, doi:10.1016/j.envint/2020.106039
Telwatte, Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1, Antiviral Res, doi:10.1016/j.antiviral.2011.03.186
Tyssen, Structure activity relationship of dendrimer microbicides with dual action antiviral activity, PLoS ONE, doi:10.1371/journal.pone.0012309
Waldbaum, A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis, PLoS ONE, doi:10.1371/journal.pone.0232394
Yan, Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community, Proc. Natl. Acad. Sci. U.S.A, doi:10.1073/pnas.1716561115
DOI record:
{
"DOI": "10.1038/s41598-022-14601-3",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-14601-3",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Astodrimer sodium is a dendrimer molecule with antiviral and virucidal activity against SARS-CoV-2 and other respiratory viruses in vitro, and has previously been shown to be safe and well tolerated, and not systemically absorbed, when applied to the vaginal mucosa. To investigate its potential utility as a topical antiviral, astodrimer sodium has been reformulated for application to the nasal mucosa to help reduce viral load before or after exposure to respiratory infection. The current investigation assessed the safety, tolerability and absorption of astodrimer sodium 1% antiviral nasal spray. This was a single-centre, double-blinded, randomized, placebo-controlled, exploratory clinical investigation. Forty healthy volunteers aged 18 to 65 years with no clinically significant nasal cavity examination findings were randomized 3:1 to astodrimer sodium nasal spray (N = 30) or placebo (N = 10) at an Australian clinical trials facility. An initial cohort of participants (N = 12 astodrimer, N = 4 placebo) received a single application (one spray per nostril) to assess any acute effects, followed by a washout period, before self-administering the spray four times daily for 14 days to represent an intensive application schedule. Extent of absorption of astodrimer sodium via the nasal mucosa was also assessed in this cohort. A second cohort of participants (N = 18 astodrimer, N = 6 placebo) self-administered the spray four times daily for 14 days. The primary endpoint was safety, measured by frequency and severity of treatment emergent adverse events (TEAEs), including clinically significant nasal cavity examination findings, in the safety population (all participants randomized who administered any spray). Participants were randomized between 6 January 2021 and 29 March 2021. TEAEs occurred in 8/10 (80%) participants in the placebo arm and 19/30 (63.3%) participants in the astodrimer sodium arm; all were of mild intensity. TEAEs considered potentially related to study product occurred in 5/10 (50%) participants receiving placebo and 10/30 (33.3%) of participants receiving astodrimer sodium. No participants experienced serious AEs, or TEAEs leading to withdrawal from the study. No systemic absorption of astodrimer sodium via the nasal mucosa was detected. Astodrimer sodium nasal spray was well tolerated and is a promising innovation warranting further investigation for nasal administration to potentially reduce infection and spread of community acquired respiratory virus infections.</jats:p><jats:p><jats:bold>Trial Registration:</jats:bold> ACTRN12620001371987, first registered 22-12-2020 (Australia New Zealand Clinical Trials Registry, <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://anzctr.org.au/\">https://anzctr.org.au/</jats:ext-link>).\n</jats:p>",
"alternative-id": [
"14601"
],
"article-number": "10210",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "9 June 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "17 June 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors JRAP, GPH and AS are paid employees of Starpharma Pty Ltd. The authors AC, CAL, GRK, PB and PMc are paid consultants to Starpharma Pty Ltd. JRAP, GPH, AS, AC and CAL are shareholders of Starpharma Holdings Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Castellarnau",
"given": "Alex",
"sequence": "first"
},
{
"affiliation": [],
"family": "Heery",
"given": "Graham P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seta",
"given": "Aynaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luscombe",
"given": "Carolyn A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinghorn",
"given": "George R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Button",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCloud",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paull",
"given": "Jeremy R. A.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "actrn12620001371987",
"registry": "10.18810/anzctr"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
6,
17
]
],
"date-time": "2022-06-17T11:26:00Z",
"timestamp": 1655465160000
},
"deposited": {
"date-parts": [
[
2022,
11,
25
]
],
"date-time": "2022-11-25T05:28:36Z",
"timestamp": 1669354116000
},
"funder": [
{
"name": "Starpharma Pty Ltd"
},
{
"award": [
"BTBR300093"
],
"name": "Australian Medical Research Future Fund Biomedical Translation Bridge Program"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
6
]
],
"date-time": "2024-09-06T10:19:03Z",
"timestamp": 1725617943517
},
"is-referenced-by-count": 9,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
6,
17
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
17
]
],
"date-time": "2022-06-17T00:00:00Z",
"timestamp": 1655424000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
17
]
],
"date-time": "2022-06-17T00:00:00Z",
"timestamp": 1655424000000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-14601-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-14601-3",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-14601-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
6,
17
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
17
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1183/13993003.01727-2020",
"author": "CWM Ong",
"doi-asserted-by": "publisher",
"first-page": "2001727",
"journal-title": "Eur. Respir. J.",
"key": "14601_CR1",
"unstructured": "Ong, C. W. M. et al. Epidemic and pandemic viral infection: Impact on tuberculosis and the lung: A consensus by the World Association for Infectious Diseases and Immunological Disorders (WAidid), Global Tuberculosis Network (GTN), and members of the European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycobacterial Infections (ESGMYC). Eur. Respir. J. 56, 2001727. https://doi.org/10.1183/13993003.01727-2020 (2020).",
"volume": "56",
"year": "2020"
},
{
"key": "14601_CR2",
"unstructured": "Centers for Disease Control and Prevention. Estimated Flu-Related Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2018–2019 flu season (2021). www.cdc.gov/flu/about/burden/2018-2019.html (Accessed 16 December 2021)."
},
{
"DOI": "10.1038/d41586-020-00974-w",
"author": "D Lewis",
"doi-asserted-by": "publisher",
"first-page": "175",
"journal-title": "Nature",
"key": "14601_CR3",
"unstructured": "Lewis, D. Is the coronavirus airborne? Experts can’t agree. Nature 580, 175. https://doi.org/10.1038/d41586-020-00974-w (2020).",
"volume": "580",
"year": "2020"
},
{
"DOI": "10.1016/j.envint/2020.106039",
"author": "S Tang",
"doi-asserted-by": "publisher",
"first-page": "106039",
"journal-title": "Environ. Int.",
"key": "14601_CR4",
"unstructured": "Tang, S. et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 144, 106039. https://doi.org/10.1016/j.envint/2020.106039 (2020).",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1716561115",
"author": "J Yan",
"doi-asserted-by": "publisher",
"first-page": "1081",
"journal-title": "Proc. Natl. Acad. Sci. U.S.A.",
"key": "14601_CR5",
"unstructured": "Yan, J. et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. U.S.A. 115, 1081–1086. https://doi.org/10.1073/pnas.1716561115 (2018).",
"volume": "115",
"year": "2018"
},
{
"DOI": "10.1021/mp050023q",
"author": "TD McCarthy",
"doi-asserted-by": "publisher",
"first-page": "312",
"journal-title": "Mol. Pharm.",
"key": "14601_CR6",
"unstructured": "McCarthy, T. D. et al. Dendrimers as drugs: Discovery and preclinical and clinical development of Dendrimer-based microbicides for HIV and STI. Mol. Pharm. 2, 312–318. https://doi.org/10.1021/mp050023q (2005).",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.1016/j.antiviral.2005.08.004",
"author": "E Gong",
"doi-asserted-by": "publisher",
"first-page": "139",
"journal-title": "Antiviral Res.",
"key": "14601_CR7",
"unstructured": "Gong, E. et al. Evaluation of Dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antiviral Res. 68, 139–146. https://doi.org/10.1016/j.antiviral.2005.08.004 (2005).",
"volume": "68",
"year": "2005"
},
{
"DOI": "10.1371/journal.pone.0012309",
"author": "D Tyssen",
"doi-asserted-by": "publisher",
"first-page": "1057",
"journal-title": "PLoS ONE",
"key": "14601_CR8",
"unstructured": "Tyssen, D. et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS ONE 5, 1057–1064. https://doi.org/10.1371/journal.pone.0012309 (2010).",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2011.03.186",
"author": "S Telwatte",
"doi-asserted-by": "publisher",
"first-page": "195",
"journal-title": "Antiviral Res.",
"key": "14601_CR9",
"unstructured": "Telwatte, S. et al. Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1. Antiviral Res. 90, 195–199. https://doi.org/10.1016/j.antiviral.2011.03.186 (2011).",
"volume": "90",
"year": "2011"
},
{
"DOI": "10.1089/aid.2005.21.207",
"author": "Y Jiang",
"doi-asserted-by": "publisher",
"first-page": "207",
"journal-title": "AIDS Res. Hum. Retroviruses",
"key": "14601_CR10",
"unstructured": "Jiang, Y. et al. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retroviruses 21, 207–213. https://doi.org/10.1089/aid.2005.21.207 (2005).",
"volume": "21",
"year": "2005"
},
{
"DOI": "10.1128/AAC.47.12.3784-3788.2003",
"author": "D Bernstein",
"doi-asserted-by": "publisher",
"first-page": "3784",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "14601_CR11",
"unstructured": "Bernstein, D. et al. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 47, 3784–3788. https://doi.org/10.1128/AAC.47.12.3784-3788.2003 (2003).",
"volume": "47",
"year": "2003"
},
{
"DOI": "10.1016/j.antiviral.2021.105089",
"author": "JRA Paull",
"doi-asserted-by": "publisher",
"first-page": "10589",
"journal-title": "Antiviral Res.",
"key": "14601_CR12",
"unstructured": "Paull, J. R. A. et al. Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro. Antiviral Res. 191, 10589. https://doi.org/10.1016/j.antiviral.2021.105089 (2021).",
"volume": "191",
"year": "2021"
},
{
"key": "14601_CR13",
"unstructured": "Gallay, P. A. et al. Effects of astodrimer sodium against SARS-CoV-2 variants (α, β, γ, δ, κ) in vitro [CROI Abstract 478]. In Special Issue: Abstracts from the virtual 2022 Conference on Retroviruses and Opportunistic Infections. Top. Antivir. Med. Vol. 30, 182 (2022)."
},
{
"DOI": "10.3390/v13081656",
"author": "JRA Paull",
"doi-asserted-by": "publisher",
"first-page": "1656",
"journal-title": "Viruses",
"key": "14601_CR14",
"unstructured": "Paull, J. R. A. et al. Protective effects of astodrimer sodium 1% nasal spray formulation against SARS-CoV-2 nasal challenge in K18-hACE2 mice. Viruses 13, 1656. https://doi.org/10.3390/v13081656 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1128/JVI.02012-06",
"author": "PB McCray",
"doi-asserted-by": "publisher",
"first-page": "813",
"journal-title": "J. Virol.",
"key": "14601_CR15",
"unstructured": "McCray, P. B. et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 81, 813–821. https://doi.org/10.1128/JVI.02012-06 (2007).",
"volume": "81",
"year": "2007"
},
{
"DOI": "10.1186/1465-9921-14-124",
"author": "M Ludwig",
"doi-asserted-by": "publisher",
"first-page": "124",
"issue": "1",
"journal-title": "Respir. Res.",
"key": "14601_CR16",
"unstructured": "Ludwig, M. et al. Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir. Res. 14(1), 124. https://doi.org/10.1186/1465-9921-14-124 (2013).",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1186/s12931-015-0281-8",
"author": "R Eccles",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Respir. Res.",
"key": "14601_CR17",
"unstructured": "Eccles, R. et al. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial. Respir. Res. 16, 121. https://doi.org/10.1186/s12931-015-0281-8 (2015).",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1371/journal.pone.0232394",
"author": "A Waldbaum",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "PLoS ONE",
"key": "14601_CR18",
"unstructured": "Waldbaum, A. et al. A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis. PLoS ONE 15, 1–13. https://doi.org/10.1371/journal.pone.0232394 (2020).",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/j.ejogrb.2019.11.032",
"author": "S Chavoustie",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "Eur. J. Obstet. Gynecol. Rep. Biol.",
"key": "14601_CR19",
"unstructured": "Chavoustie, S. et al. Two phase 3, double-blind, placebo-controlled studies of the efficacy and safety of Astodrimer 1% Gel for the treatment of bacterial vaginosis. Eur. J. Obstet. Gynecol. Rep. Biol. 245, 13–18. https://doi.org/10.1016/j.ejogrb.2019.11.032 (2020).",
"volume": "245",
"year": "2020"
},
{
"DOI": "10.1016/j.eurox.2021.100121",
"author": "J Schwebke",
"doi-asserted-by": "publisher",
"first-page": "100121",
"journal-title": "Eur. J. Obstet. Gynecol. Rep. Biol. X",
"key": "14601_CR20",
"unstructured": "Schwebke, J. et al. A phase 3, randomized, controlled trial of Astodrimer 1% Gel for preventing recurrent bacterial vaginosis. Eur. J. Obstet. Gynecol. Rep. Biol. X 10, 100121. https://doi.org/10.1016/j.eurox.2021.100121 (2021).",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1097/OLQ.0b013e3181bc0aac",
"author": "J O’Loughlin",
"doi-asserted-by": "publisher",
"first-page": "100",
"journal-title": "Sex Transm. Dis.",
"key": "14601_CR21",
"unstructured": "O’Loughlin, J. et al. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel®): A dose ranging phase 1 study. Sex Transm. Dis. 37, 100–104. https://doi.org/10.1097/OLQ.0b013e3181bc0aac (2010).",
"volume": "37",
"year": "2010"
},
{
"DOI": "10.1097/QAD.0b013e328346bd3e",
"author": "I McGowan",
"doi-asserted-by": "publisher",
"first-page": "1057",
"journal-title": "AIDS",
"key": "14601_CR22",
"unstructured": "McGowan, I. et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel®) in sexually active young women (MTN-004). AIDS 25, 1057–1064. https://doi.org/10.1097/QAD.0b013e328346bd3e (2011).",
"volume": "25",
"year": "2011"
},
{
"DOI": "10.1371/journal.ppat.1010079",
"author": "N Ratcliffe",
"doi-asserted-by": "publisher",
"first-page": "e1010079",
"journal-title": "PLoS Pathog.",
"key": "14601_CR23",
"unstructured": "Ratcliffe, N. et al. Nasal therapy—The missing link in optimising strategies to improve prevention and treatment of COVID-19. PLoS Pathog. 17, e1010079. https://doi.org/10.1371/journal.ppat.1010079 (2021).",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S167123",
"author": "C Graf",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "Int. J. Gen. Med.",
"key": "14601_CR24",
"unstructured": "Graf, C. et al. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int. J. Gen. Med. 11, 275–283. https://doi.org/10.2147/IJGM.S167123 (2018).",
"volume": "11",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0024095",
"author": "CF Price",
"doi-asserted-by": "publisher",
"first-page": "e24095",
"issue": "9",
"journal-title": "PLoS ONE",
"key": "14601_CR25",
"unstructured": "Price, C. F. et al. SPL7013 (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS ONE 6(9), e24095. https://doi.org/10.1371/journal.pone.0024095 (2011).",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1111/ijcp.14165",
"author": "A Abu-Zaid",
"doi-asserted-by": "publisher",
"first-page": "e14165",
"journal-title": "Int. J. Clin. Pract.",
"key": "14601_CR26",
"unstructured": "Abu-Zaid, A. et al. Astodrimer gel for treatment of bacterial vaginosis: A systematic review and meta-analysis of randomized controlled trials. Int. J. Clin. Pract. 75, e14165. https://doi.org/10.1111/ijcp.14165 (2021).",
"volume": "75",
"year": "2021"
},
{
"key": "14601_CR27",
"unstructured": "World Health Organization. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions (2020). www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (Accessed 21 December 2021)."
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-14601-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}