Avdoralimab (Anti-C5aR1 mAb) Versus Placebo in Patients With Severe COVID-19: Results From a Randomized Controlled Trial (FOR COVID Elimination [FORCE])
et al., Critical Care Medicine, doi:10.1097/CCM.0000000000005683, FORCE, Oct 2022
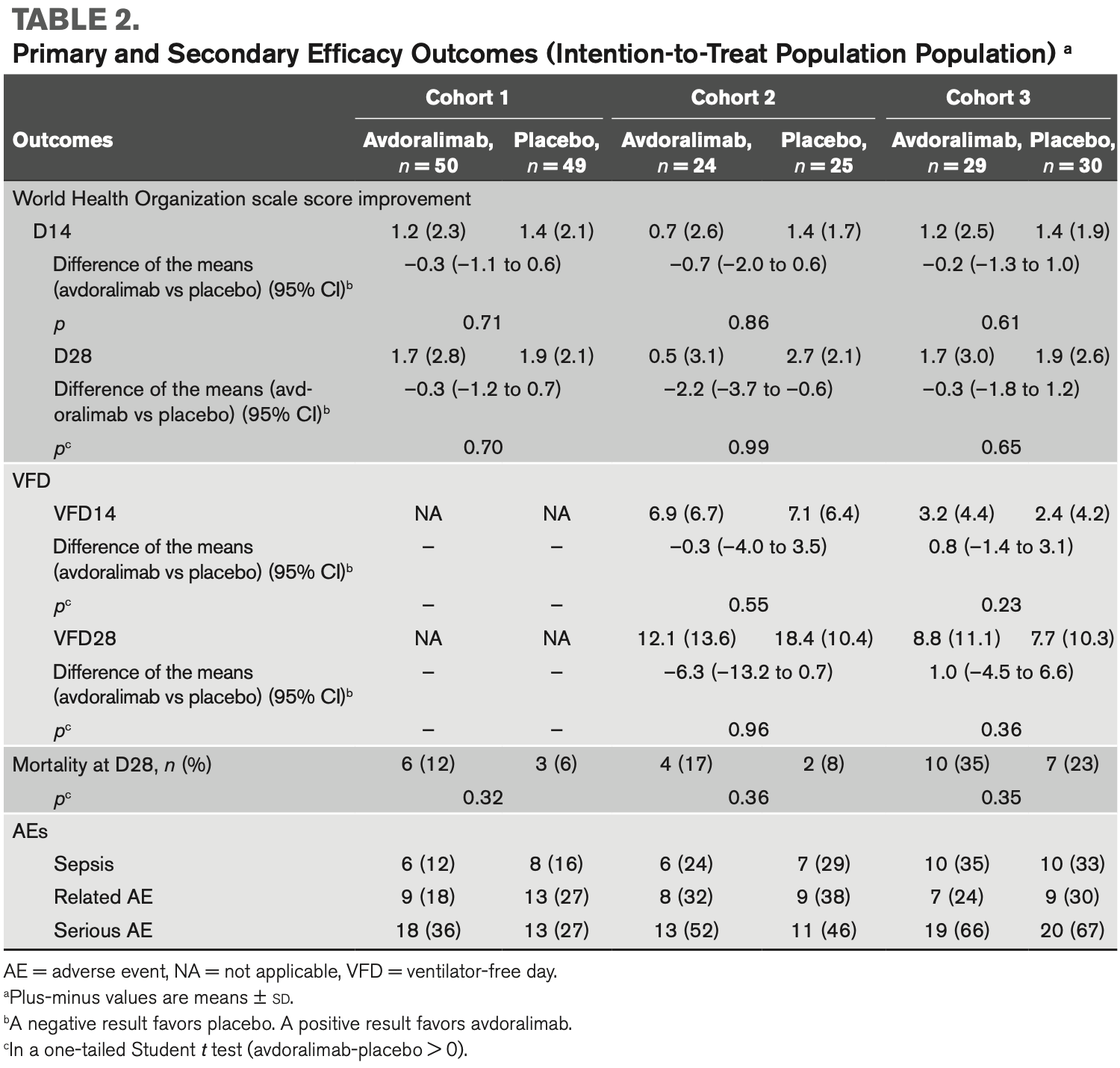
RCT 207 hospitalized COVID-19 patients showing no significant benefit with avdoralimab, a C5a receptor (C5aR1) inhibitor, compared to placebo across three cohorts of patients with varying COVID-19 severity.
|
risk of death, 68.3% higher, RR 1.68, p = 0.13, treatment 20 of 103 (19.4%), control 12 of 104 (11.5%).
|
|
risk of death, 96.0% higher, RR 1.96, p = 0.49, treatment 6 of 50 (12.0%), control 3 of 49 (6.1%), cohort 1.
|
|
risk of death, 108.3% higher, RR 2.08, p = 0.42, treatment 4 of 24 (16.7%), control 2 of 25 (8.0%), cohort 2.
|
|
risk of death, 47.8% higher, RR 1.48, p = 0.40, treatment 10 of 29 (34.5%), control 7 of 30 (23.3%), cohort 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Carvelli et al., 10 Oct 2022, Double Blind Randomized Controlled Trial, placebo-controlled, France, peer-reviewed, 27 authors, average treatment delay 9.8 days, FORCE trial.
Abstract: Avdoralimab (Anti-C5aR1 mAb) Versus
Placebo in Patients With Severe COVID-19:
Results From a Randomized Controlled Trial
(FOR COVID Elimination [FORCE])*
OBJECTIVES: Severe COVID-19 is associated with exaggerated complement
activation. We assessed the efficacy and safety of avdoralimab (an anti-C5aR1
mAb) in severe COVID-19.
DESIGN: FOR COVID Elimination (FORCE) was a double-blind, placebo-controlled study.
SETTING: Twelve clinical sites in France (ICU and general hospitals).
PATIENTS: Patients receiving greater than or equal to 5 L oxygen/min to maintain
Spo2 greater than 93% (World Health Organization scale ≥ 5). Patients received
conventional oxygen therapy or high-flow oxygen (HFO)/noninvasive ventilation
(NIV) in cohort 1; HFO, NIV, or invasive mechanical ventilation (IMV) in cohort 2;
and IMV in cohort 3.
INTERVENTIONS: Patients were randomly assigned, in a 1:1 ratio, to receive
avdoralimab or placebo. The primary outcome was clinical status on the World
Health Organization ordinal scale at days 14 and 28 for cohorts 1 and 3, and the
number of ventilator-free days at day 28 (VFD28) for cohort 2.
MEASUREMENTS AND MAIN RESULTS: We randomized 207 patients: 99
in cohort 1, 49 in cohort 2, and 59 in cohort 3. During hospitalization, 95% of
patients received glucocorticoids. Avdoralimab did not improve World Health
Organization clinical scale score on days 14 and 28 (between-group difference on day 28 of –0.26 (95% CI, –1.2 to 0.7; p = 0.7) in cohort 1 and –0.28
(95% CI, –1.8 to 1.2; p = 0.6) in cohort 3). Avdoralimab did not improve VFD28
in cohort 2 (between-group difference of –6.3 (95% CI, –13.2 to 0.7; p = 0.96)
or secondary outcomes in any cohort. No subgroup of interest was identified.
CONCLUSIONS: In this randomized trial in hospitalized patients with severe COVID19 pneumonia, avdoralimab did not significantly improve clinical status at days 14
and 28 (funded by Innate Pharma, ClinicalTrials.gov number, NCT04371367).
KEY WORDS: avdoralimab; complement; COVID-19; inflammation; sepsis
S
ince its emergence in China in 2019, the original strain of severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants have
infected ~260 million people globally, and over 5 million people worldwide had died from COVID-19 by the end of 2021 (1). With the progression of
the pandemic, very few treatment options have proved effective, and COVID19 continues to be a major public health problem. In 10–20% of hospitalized
patients, transfer to an ICU is required, due to acute respiratory distress syndrome, requiring high-flow oxygen (HFO), noninvasive ventilation (NIV), or
invasive mechanical ventilation (IMV) (2, 3). Severe COVID-19 is characterized by overt inflammation of the lungs in response to the viral infection (4–6).
1788 www.ccmjournal.org
Julien Carvelli, MD1–3
Ferhat Meziani, MD, PhD4
Jean Dellamonica, MD, PhD5
Pierre-Yves Cordier, MD6
Jerome Allardet-Servent, MD7
Megan Fraisse, MD8
Lionel Velly, MD, PhD3,9
Saber Davide Barbar, MD, PhD10
Samuel Lehingue, MD11,
Christophe Guervilly, MD3,12
Maxime Desgrouas, MD13
Fabrice Camou, MD, PhD14
Christelle Piperoglou2,15
Frederic Vely, PhD2,3,15
Olivier Demaria, PhD16
Joyson Karakunnel, MD16
Joanna Fares, PhD16
Luciana Batista, PhD16
Federico Rotolo, PhD16
Julien Viotti, PhD16
Agnes Boyer-Chammard, MD16
Karine Lacombe, MD, PhD17
Erwan Le Dault, PhD18
Michel Carles, MD, PhD19
Nicolas Schleinitz, MD, PhD2,3,20
Eric Vivier, DVM, PhD2,3,15,16
for the FOR COVID Elimination
(FORCE)..
DOI record:
{
"DOI": "10.1097/ccm.0000000000005683",
"ISSN": [
"0090-3493"
],
"URL": "http://dx.doi.org/10.1097/CCM.0000000000005683",
"abstract": "<jats:sec>\n <jats:title>OBJECTIVES:</jats:title>\n <jats:p>Severe COVID-19 is associated with exaggerated complement activation. We assessed the efficacy and safety of avdoralimab (an anti-C5aR1 mAb) in severe COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>DESIGN:</jats:title>\n <jats:p>FOR COVID Elimination (FORCE) was a double-blind, placebo-controlled study.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>SETTING:</jats:title>\n <jats:p>Twelve clinical sites in France (ICU and general hospitals).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>PATIENTS:</jats:title>\n <jats:p>Patients receiving greater than or equal to 5 L oxygen/min to maintain Sp<jats:sc>o</jats:sc>\n <jats:sub>2</jats:sub> greater than 93% (World Health Organization scale ≥ 5). Patients received conventional oxygen therapy or high-flow oxygen (HFO)/noninvasive ventilation (NIV) in cohort 1; HFO, NIV, or invasive mechanical ventilation (IMV) in cohort 2; and IMV in cohort 3.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>INTERVENTIONS:</jats:title>\n <jats:p>Patients were randomly assigned, in a 1:1 ratio, to receive avdoralimab or placebo. The primary outcome was clinical status on the World Health Organization ordinal scale at days 14 and 28 for cohorts 1 and 3, and the number of ventilator-free days at day 28 (VFD28) for cohort 2.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>MEASUREMENTS AND MAIN RESULTS:</jats:title>\n <jats:p>We randomized 207 patients: 99 in cohort 1, 49 in cohort 2, and 59 in cohort 3. During hospitalization, 95% of patients received glucocorticoids. Avdoralimab did not improve World Health Organization clinical scale score on days 14 and 28 (between-group difference on day 28 of –0.26 (95% CI, –1.2 to 0.7; <jats:italic toggle=\"yes\">p</jats:italic> = 0.7) in cohort 1 and –0.28 (95% CI, –1.8 to 1.2; <jats:italic toggle=\"yes\">p</jats:italic> = 0.6) in cohort 3). Avdoralimab did not improve VFD28 in cohort 2 (between-group difference of –6.3 (95% CI, –13.2 to 0.7; <jats:italic toggle=\"yes\">p</jats:italic> = 0.96) or secondary outcomes in any cohort. No subgroup of interest was identified.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>CONCLUSIONS:</jats:title>\n <jats:p>In this randomized trial in hospitalized patients with severe COVID-19 pneumonia, avdoralimab did not significantly improve clinical status at days 14 and 28 (funded by Innate Pharma, ClinicalTrials.gov number, NCT04371367).</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [],
"family": "Carvelli",
"given": "Julien",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Médecine Intensive & Réanimation, Nouvel Hôpital Civil, Strasbourg University Hospital, INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine (RNM), FMTS (Fédération de Médecine Translationnelle de Strasbourg), Strasbourg University, Strasbourg, France."
}
],
"family": "Meziani",
"given": "Ferhat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Medecine Intensive & Reanimation, Hôpital l’Archet 1, Nice University Hospital, Cote d’Azur University, UR2CA, Nice, France."
}
],
"family": "Dellamonica",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Réanimation Polyvalente, HIA Laveran, Marseille, France."
}
],
"family": "Cordier",
"given": "Pierre-Yves",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Hôpital Européen, Marseille, France."
}
],
"family": "Allardet-Servent",
"given": "Jerome",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Réanimation Polyvalente, Centre Hospitalier Victor Dupouy, Argenteuil, France."
}
],
"family": "Fraisse",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aix-Marseille University, Marseille, France."
},
{
"name": "AP-HM, Department of Anesthesiology & Critical Care Medicine, Réanimation Polyvalente, Timone University Hospital, Institut Neuroscience Timone CNRS UMR-7289, Marseille, France."
}
],
"family": "Velly",
"given": "Lionel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Nîmes University Hospital, University of Montpellier, Nîmes, France."
}
],
"family": "Barbar",
"given": "Saber Davide",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Hôpital Saint-Joseph, Marseille, France."
}
],
"family": "Lehingue",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aix-Marseille University, Marseille, France."
},
{
"name": "AP-HM, Department of Intensive Care, Médecine Intensive Réanimation, North Hospital, Centre d’Etudes et de Recherches sur les Services de Santé et qualité de vie EA 3279Marseille, Marseille, France."
}
],
"family": "Guervilly",
"given": "Christophe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Medecine Intensive & Reanimation, Centre Hospitalier Régional d’Orléans, Orléans, France."
}
],
"family": "Desgrouas",
"given": "Maxime",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care, Bordeaux University Hospital, Hôpital Saint-André, University of Bordeaux, Bordeaux, France."
}
],
"family": "Camou",
"given": "Fabrice",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Marseille Immunopôle, Timone University Hospital, CNRS, INSERM, CIML, Marseille, France."
},
{
"name": "AP-HM, Timone University Hospital, Immunology, Marseille, France."
}
],
"family": "Piperoglou",
"given": "Christelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Marseille Immunopôle, Timone University Hospital, CNRS, INSERM, CIML, Marseille, France."
},
{
"name": "Aix-Marseille University, Marseille, France."
},
{
"name": "AP-HM, Timone University Hospital, Immunology, Marseille, France."
}
],
"family": "Vely",
"given": "Frederic",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Demaria",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Karakunnel",
"given": "Joyson",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Fares",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Batista",
"given": "Luciana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Rotolo",
"given": "Federico",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Viotti",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Boyer-Chammard",
"given": "Agnes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AP-HP, Tropical Medicine & Infectious Diseases, Saint-Antoine Univeristy Hospital, Sorbonne University, Inserm UMR-S1136 IPLESP, Paris, France."
}
],
"family": "Lacombe",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tropical Medicine & Infectious Diseases, HIA Laveran, Marseille, France."
}
],
"family": "Le Dault",
"given": "Erwan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tropical Medicine & Infectious Diseases, Hôpital l’Archet 1, Nice Univeristy Hospital, Université Côte d’Azur, Inserm-C3M, Nice, France."
}
],
"family": "Carles",
"given": "Michel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Marseille Immunopôle, Timone University Hospital, CNRS, INSERM, CIML, Marseille, France."
},
{
"name": "Aix-Marseille University, Marseille, France."
},
{
"name": "AP-HM, Internal Medicine Department, Timone University Hospital, Marseille, France."
}
],
"family": "Schleinitz",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Marseille Immunopôle, Timone University Hospital, CNRS, INSERM, CIML, Marseille, France."
},
{
"name": "Aix-Marseille University, Marseille, France."
},
{
"name": "AP-HM, Timone University Hospital, Immunology, Marseille, France."
},
{
"name": "Innate Pharma, Marseille, France."
}
],
"family": "Vivier",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the FOR COVID Elimination (FORCE) Study Group",
"sequence": "additional"
}
],
"container-title": "Critical Care Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"lww.com",
"ovid.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
11
]
],
"date-time": "2022-10-11T13:06:35Z",
"timestamp": 1665493595000
},
"deposited": {
"date-parts": [
[
2024,
8,
6
]
],
"date-time": "2024-08-06T05:26:12Z",
"timestamp": 1722921972000
},
"indexed": {
"date-parts": [
[
2025,
4,
15
]
],
"date-time": "2025-04-15T16:29:42Z",
"timestamp": 1744734582750
},
"is-referenced-by-count": 21,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
10,
10
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T00:00:00Z",
"timestamp": 1665360000000
}
}
],
"link": [
{
"URL": "https://journals.lww.com/10.1097/CCM.0000000000005683",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "1788-1798",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2022,
10,
10
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1016/S2213-2600(20)30527-0",
"article-title": "Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study.",
"author": "Piroth",
"doi-asserted-by": "crossref",
"first-page": "251",
"journal-title": "Lancet Respir Med",
"key": "R2-20240805",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1186/s13613-021-00933-2",
"article-title": "Impact of ICU transfers on the mortality rate of patients with COVID-19: Insights from comprehensive national database in France.",
"author": "Sanchez",
"doi-asserted-by": "crossref",
"first-page": "151",
"journal-title": "Ann Intensive Care",
"key": "R3-20240805",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-03148-w",
"article-title": "Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia.",
"author": "Grant",
"doi-asserted-by": "crossref",
"first-page": "635",
"journal-title": "Nature",
"key": "R4-20240805",
"volume": "590",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03570-8",
"article-title": "COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets.",
"author": "Delorey",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Nature",
"key": "R5-20240805",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03475-6",
"article-title": "The spatial landscape of lung pathology during COVID-19 progression.",
"author": "Rendeiro",
"doi-asserted-by": "crossref",
"first-page": "564",
"journal-title": "Nature",
"key": "R6-20240805",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for covid-19 — interim WHO solidarity trial results",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "R7-20240805",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19.",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "R8-20240805",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial.",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "R9-20240805",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with Covid-19.",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "1491",
"journal-title": "N Engl J Med",
"key": "R10-20240805",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01499-z",
"article-title": "Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial.",
"author": "Kyriazopoulou",
"doi-asserted-by": "crossref",
"first-page": "1752",
"journal-title": "Nat Med",
"key": "R11-20240805",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial.",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir Med",
"key": "R12-20240805",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2101643",
"article-title": "Tofacitinib in patients hospitalized with Covid-19 pneumonia",
"author": "Guimarães",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "N Engl J Med",
"key": "R13-20240805",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.lanepe.2021.100243",
"article-title": "Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study.",
"author": "Carbonell",
"doi-asserted-by": "crossref",
"first-page": "100243",
"journal-title": "Lancet Reg Health Eur",
"key": "R14-20240805",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1126/sciimmunol.abg0833",
"article-title": "SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation.",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "eabg0833",
"journal-title": "Sci Immunol",
"key": "R15-20240805",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.05.032",
"article-title": "Proteomic and metabolomic characterization of covid-19 patient sera.",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "59",
"journal-title": "Cell",
"key": "R16-20240805",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1021-2",
"article-title": "Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection.",
"author": "Ramlall",
"doi-asserted-by": "crossref",
"first-page": "1609",
"journal-title": "Nat Med",
"key": "R17-20240805",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/978-1-4614-0106-3_9",
"article-title": "Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis.",
"author": "Bosmann",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "Adv Exp Med Biol",
"key": "R18-20240805",
"volume": "946",
"year": "2012"
},
{
"DOI": "10.1096/fj.15-271635",
"article-title": "Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): A role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin.",
"author": "Russkamp",
"doi-asserted-by": "crossref",
"first-page": "3762",
"journal-title": "FASEB J",
"key": "R19-20240805",
"volume": "29",
"year": "2015"
},
{
"DOI": "10.1038/s41586-020-2600-6",
"article-title": "Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis.",
"author": "Carvelli",
"doi-asserted-by": "crossref",
"first-page": "146",
"journal-title": "Nature",
"key": "R20-20240805",
"volume": "588",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30341-6",
"article-title": "Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): An exploratory, open-label, phase 2 randomised controlled trial.",
"author": "Vlaar",
"doi-asserted-by": "crossref",
"first-page": "e764",
"journal-title": "Lancet Rheumatol",
"key": "R21-20240805",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100590",
"article-title": "Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study.",
"author": "Annane",
"doi-asserted-by": "crossref",
"first-page": "100590",
"journal-title": "EClinicalMedicine",
"key": "R22-20240805",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1164/rccm.201810-2050CP",
"article-title": "Reappraisal of ventilator-free days in critical care research.",
"author": "Yehya",
"doi-asserted-by": "crossref",
"first-page": "828",
"journal-title": "Am J Respir Crit Care Med",
"key": "R23-20240805",
"volume": "200",
"year": "2019"
},
{
"DOI": "10.1001/jama.2016.0287",
"article-title": "The third international consensus definitions for sepsis and septic shock (Sepsis-3).",
"author": "Singer",
"doi-asserted-by": "crossref",
"first-page": "801",
"journal-title": "JAMA",
"key": "R24-20240805",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1002/sim.6911",
"article-title": "Fallback tests for co-primary endpoints.",
"author": "Ristl",
"doi-asserted-by": "crossref",
"first-page": "2669",
"journal-title": "Stat Med",
"key": "R25-20240805",
"volume": "35",
"year": "2016"
},
{
"DOI": "10.3389/fimmu.2021.714511",
"article-title": "Lectin pathway mediates complement activation by SARS-CoV-2 proteins.",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "714511",
"journal-title": "Front Immunol",
"key": "R26-20240805",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1182/blood.2020008248",
"article-title": "Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition.",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "2080",
"journal-title": "Blood",
"key": "R27-20240805",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06294-x",
"article-title": "Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study.",
"doi-asserted-by": "crossref",
"first-page": "60",
"journal-title": "Intensive Care Med",
"key": "R28-20240805",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(21)00321-0",
"article-title": "IL-1 and IL-6 inhibition affects the neutralising activity of anti-SARS-CoV-2 antibodies in patients with COVID-19.",
"author": "Della-Torre",
"doi-asserted-by": "crossref",
"first-page": "e829",
"journal-title": "Lancet Rheumatol",
"key": "R29-20240805",
"volume": "3",
"year": "2021"
},
{
"article-title": "Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study.",
"author": "Gangneux",
"first-page": "00442",
"journal-title": "Lancet Respir Med",
"key": "R30-20240805",
"volume": "S2213-2600",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/CCM.0000000000005683"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Avdoralimab (Anti-C5aR1 mAb) Versus Placebo in Patients With Severe COVID-19: Results From a Randomized Controlled Trial (FOR COVID Elimination [FORCE])*",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1097/lww.0000000000001000",
"volume": "50"
}