Metformin reduces the risk of Long COVID or Death over 6 months in an Emulated Target Trial of Primarily Omicron-infected Adults without Diabetes or Prediabetes: a New-User, Active-Comparator Analysis Using the National COVID Cohort Collaborative (N3C) Electronic Health Record Database.
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofae631.016, Jan 2025
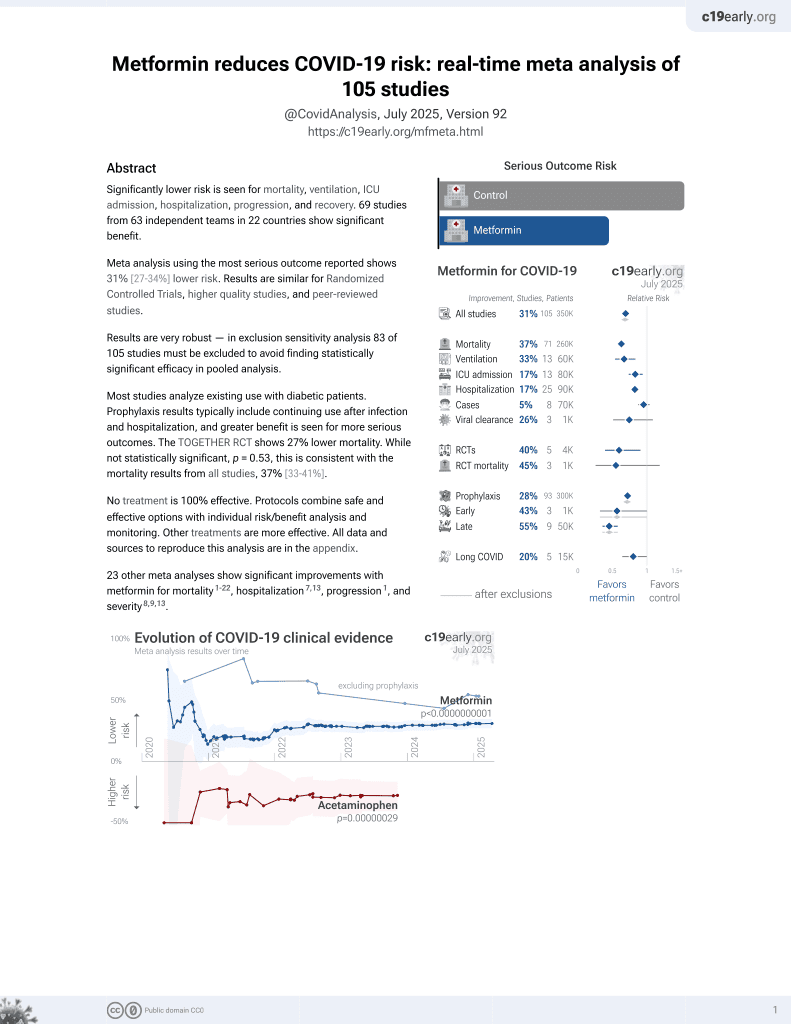
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Emulated target trial of Omicron-infected outpatients without diabetes or prediabetes, showing significantly lower long COVID or death with metformin treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
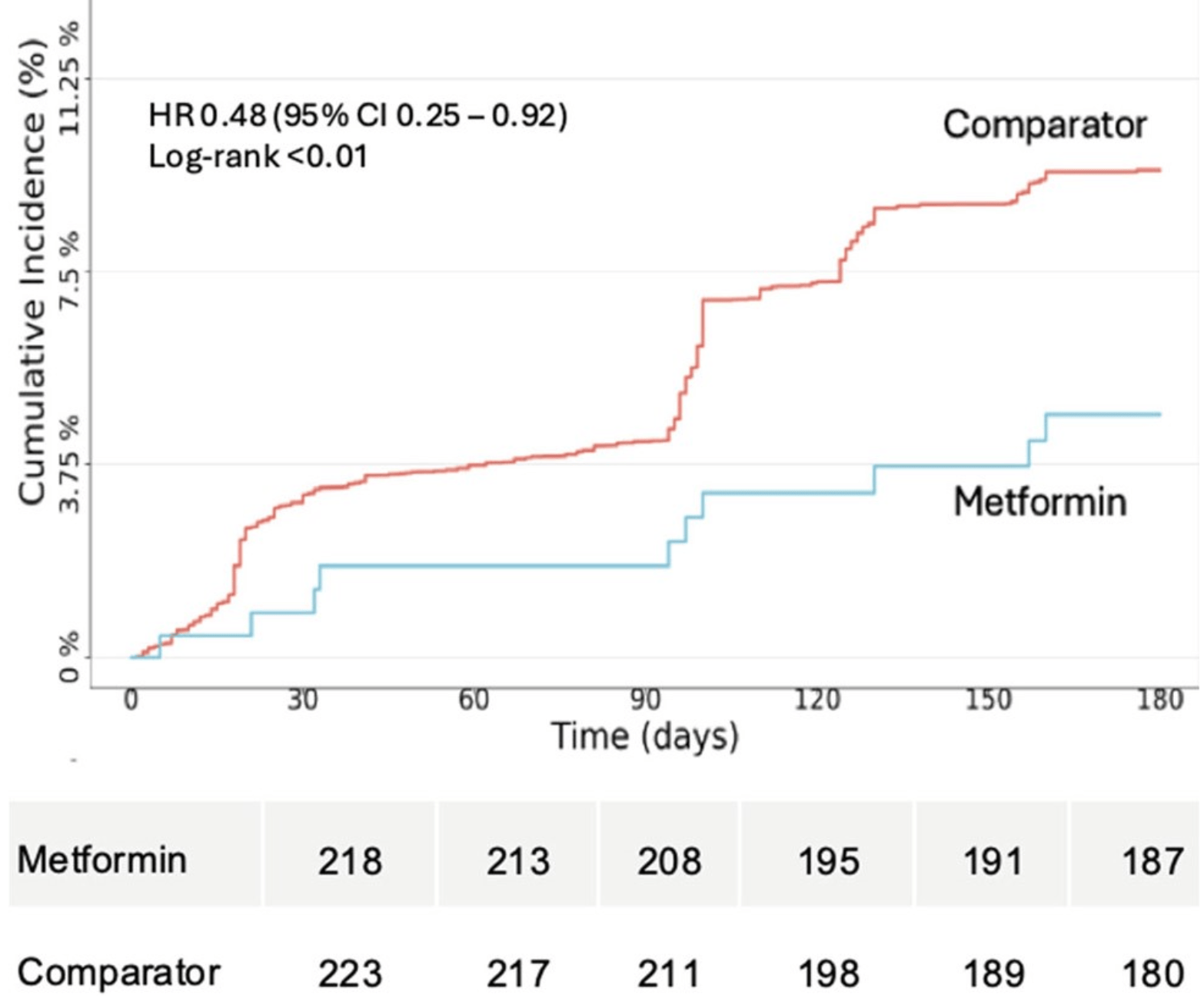
long COVID or death, 53.0% lower, HR 0.47, p = 0.02, treatment 10 of 248 (4.0%), control 21 of 248 (8.5%), NNT 23.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bramante et al., 29 Jan 2025, retrospective, USA, peer-reviewed, 10 authors.
Abstract: PhD6; 1University of Minnesota, Minneapolis, MN; 2University of North Carolina,
Chapel Hill, Chapel Hill, North Carolina; 3Duke University, Durham, North Carolina;
4
University of Virginia, Charlottesville, Virginia; 5National Institute of Health,
Bethesda, Maryland; 6Scripps Research, San Diego, California
Study Group: n/a
Session: 253. Late Breaker Abstract Session: What’s Going Viral
Saturday, October 19, 2024: 2:21 PM
3
/µL; P=0.23).
Cumulative incidence curves for the outcome of Long Covid/Death in the 180
Days after a metformin prescription (blue) or active comparator prescription (red).
The increase in outcomes at Day 90 reflects the computable phenotype, which
cannot be computed until 90 days after infection. Per data use requirements in the
National Covid Cohort Collaborative (N3C), the starting number at risk is not
shown due to small cell sizes or the ability to calculate small cell sizes.
Methods. We emulated a randomized trial of metformin vs. control in
SARS-CoV-2-infected outpatients. Intervention: prescription for metformin within
6 days of infection. Control: prescription for fluvoxamine, fluticasone, ivermectin,
or montelukast (drugs used off-label for Covid but clinical trials have shown no effect
on acute COVID outcomes). Exclusions: age < 18; metformin or comparator prescribed within 365 days; indication for chronic metformin use; contraindication for
metformin or control. Outcome: a composite of LC or Death (LC/D), LC defined
by U09.9 or a symptom/condition based computable phenotype. We used entropy
balancing to estimate the average treatment effect with a weighted log linear model.
This is a forest plot showing metformin use versus active comparator use and the
development of Long Covid/Death by Day 180.
Abstract citation ID: ofae631.016
578. Metformin reduces the risk of Long COVID or Death over 6 months in an
Emulated Target Trial of Primarily Omicron-infected Adults without Diabetes or
Prediabetes: a New-User, Active-Comparator Analysis Using the National COVID
Cohort Collaborative (N3C) Electronic Health Record Database. This research was
supported in part by the Intramural/Extramural research program of the National
Center for Advancing Translational Science, NIH
Carolyn Bramante, MD, MPH1; Til Sturmer, MD, PhD2; Jared Huling, PhD1;
John Buse, MD, PhD2; Steve Johnson, PhD1; Christopher Lindsell, PhD3;
Thomas Stewart, PhD4; David Sahner, MD5; Sarah Dunsmore, PhD5; Eric Topol, MD,
S10 • OFID 2025:12 (Suppl 1) • Late Breaking Abstracts
The black squares represent risk ratios (RR), and the lines represent 95%
confidence intervals. The number of outcomes, denominators, and exact
percentages are sometimes omitted so that cell sizes <10 are not able to be
calculated, in accordance with the data use requirements in the National Covid
Cohort Collaborative (N3C). The Pre-Omicron Era is larger than the Omicron era
in both the Metformin and Comparator Cohorts, but the denominator for the Day
0-6 sample is not shown because of cell sizes <10. The subgroup of those with new
Background. Metformin has decreased SARS-CoV-2 RNA in 4 cell lines. In a
RCT of > 1,000 majority vaccinated outpatients, COVID-19 related ED visits/
Hospitalization/Death occurred in 4.7% of the metformin vs 9.0% of the placebo
group by Day 28; clinician-diagnosed Long Covid (LC) occurred in 6.2% of metformin
vs 10.3% of placebo by..