Effectiveness of Inhaled Steroids in Post COVID Cough
et al., International Journal of Scientific Development and Research, 7:3, Mar 2022
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
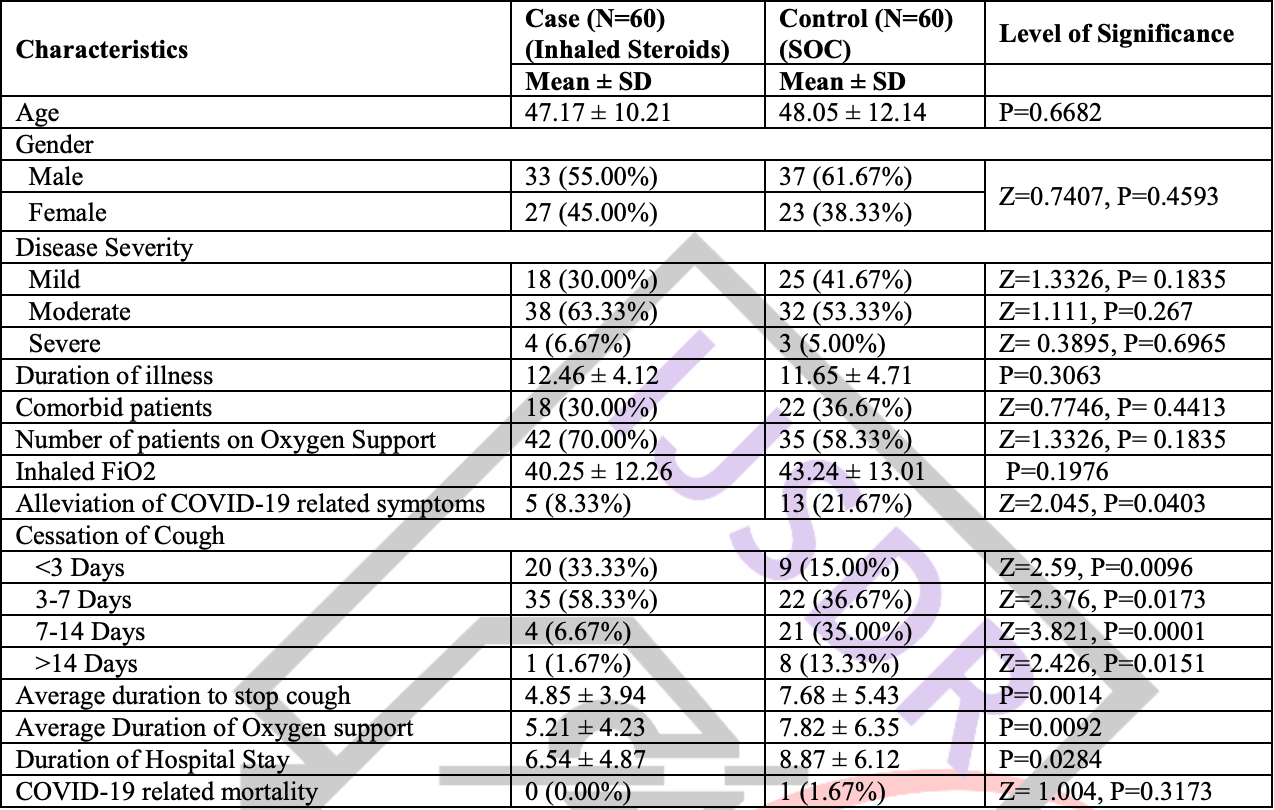
Retrospective 120 hospitalized COVID-19 patients with persistent cough in India, showing faster resolution of cough, shorter duration of oxygen support, and shorter hospitalization with inhaled budesonide treatment compared to standard of care alone.
|
risk of death, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 60 (0.0%), control 1 of 60 (1.7%), NNT 60, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
oxygen time, 33.4% lower, relative time 0.67, p = 0.009, treatment mean 5.21 (±4.23) n=60, control mean 7.82 (±6.35) n=60.
|
|
hospitalization time, 26.3% lower, relative time 0.74, p = 0.02, treatment mean 6.54 (±4.87) n=60, control mean 8.87 (±6.12) n=60.
|
|
recovery time, 36.8% lower, relative time 0.63, p = 0.001, treatment mean 4.85 (±3.94) n=60, control mean 7.68 (±5.43) n=60, cough.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bhandari et al., 22 Mar 2022, retrospective, India, peer-reviewed, 3 authors.
Effectiveness of Inhaled Steroids in Post COVID Cough
Background: Coronavirus disease 2019 can leads to persistent cough which may need special attention for better compliance of patients. We aimed to discuss the effect of inhaled steroids (Budesonide) in treatment of post COVID persistent cough and to know role of inhaled steroids in early recovery of COVID-19 patients. Methods: This retrospective observational case-control study included a total of 120 admitted patients of COVID-19 complaining of persistent cough after exclusion of another known identifiable causes. 60 patients had history of inhaled steroids for treatment of persistent cough while another 60 patients treated with standard of care treatment taken as a control group. The patient's data concerning demography, clinical profile, severity of disease, duration of illness, oxygen support, and outcome were extracted from their medical records. All collected data were tabulated, compiled, and analyzed to establish the possible causality of pneumothorax. Results: Patients of both groups had matched demographic, clinical symptoms, and comorbid status. Patients treated with inhaled steroids along with standard of care treatment had significant fast recovery (4.85 ± 3.94 days) as compared to control group (7.68 ± 5.43 days) with P=0.0014. Patients of inhaled steroids group also had early wean off from oxygen support (5.21 v/s 7.82 days), early discharge from hospital (6.54 days' v/s 8.87 days), and lesser alleviation of COVID-19 symptoms (8.33% v/s 21.67%) with P<0.05
Conclusion: This study concluded that the inhaled budesonide along with standard of care treatment helpful to relieve post COVID persistent cough. Beside early relief in cough, inhaled steroids also useful in early withdrawal of oxygen support and early discharge from hospital.
Discussion: In this study, we evaluate the effectiveness of inhaled steroids in the treatment of persistent cough after COVID-19 infection. We also try to establish an association between use of inhaled steroids and recovery from post COVID Cough. This is a retrospective case-control observational study that includes an age-matched, gender-matched, disease severity matched and underlying chronic medical illness matched control group in order to avoid these confounding factors for pneumothorax. Explaining the exact association between COVID-19 and post COVID cough is more challenging. In this study, we found that the inhaled glucocorticoid budesonide, given for the persistent cough, might be an effective treatment for post infectious cough in COVID-19 infected patients. Inhaled budesonide is a simple, safe, well studied, inexpensive, and widely available treatment. This is also significantly helpful in in low-income and middle-income countries where the majority of currently approved COVID-19 treatments are unlikely to ever reach patients as a consequence of variable health-care systems (17) . Although systemic steroids are well documented for treatment of moderate to critical COVID-19 infection but their role is not approved for use in post infectious cough. Furthermore, inhaled budesonide could work as an adjunct to reduce pressure on health-care systems and symptomatically to felt better to patients. In this study, our eyes focused on inhaled budesonide because of the..
References
Bardin, Fraenkel, Sanderson, Lampe, Holgate, Lower airways inflammatory response during rhinovirus colds, Int Arch Allergy Immunol
Braman, Postinfectious cough: ACCP evidence-based clinical practice guidelines, Chest
Chen, Zhou, Dong, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Edejer, Hanssen, Mirelman, Projected health-care resource needs for an effective response to COVID-19 in 73 low-income and middle-income countries: a modelling study, Lancet Glob Health
French, Fletcher, Irwin, A comparison of gender differences in health-related quality of life in acute and chronic coughers, Chest
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Johnstone, Chang, Fong, Bowman, Yang, Inhaled corticosteroids for subacute and chronic cough in adults, Cochrane Database Syst Rev, doi:10.1002/14651858
Kardos, Berck, Fuchs, Gillissen, Klimek et al., Guidelines of the German Respiratory Society for diagnosis and treatment of adults suffering from acute or chronic cough, Pneumologie
Matsuyama, Kawase, Nao, The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, J Virol
Nicolau, Bafadhel, Inhaled corticosteroids in virus pandemics: a treatment for COVID-19?, Lancet Respir Med
Peters, Sajuthi, Deford, COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids, Am J Respir Crit Care Med
Ponsioen, Hop, Vermue, Dekhuijzen, Bohnen, Efficacy of fluticasone on cough: a randomised controlled trial, Eur Respir J
Pornsuriyasak, Charoenpan, Vongvivat, Thakkinstian, Inhaled corticosteroid for persistent cough following upper respiratory tract infection, Respirology
Rosendal, Carlsen, Rask, Moth, Symptoms as the main problem in primary care: a cross-sectional study of frequency and characteristics, Scand J Prim Health Care
Skevaki, Karsonova, Karaulov, Xie, Renz, Asthma-associated risk for COVID-19 development, J Allergy Clin Immunol
Thorlund, Dron, Park, Hsu, Forrest et al., A real-time dashboard of clinical trials for COVID-19, Lancet Digit Health
Trigg, Nicholson, Wang, Ireland, Jordan et al., Bronchial inflammation and the common cold: a comparison of atopic and non-atopic individuals, Clin Exp Allergy
