Effect of Andrographis paniculata Treatment for Nonimmune Patients with Early-Stage COVID-19 on the Prevention of Pneumonia: A Retrospective Cohort Study
et al., Archives of Internal Medicine Research, doi:10.26502/aimr.0146, May 2023
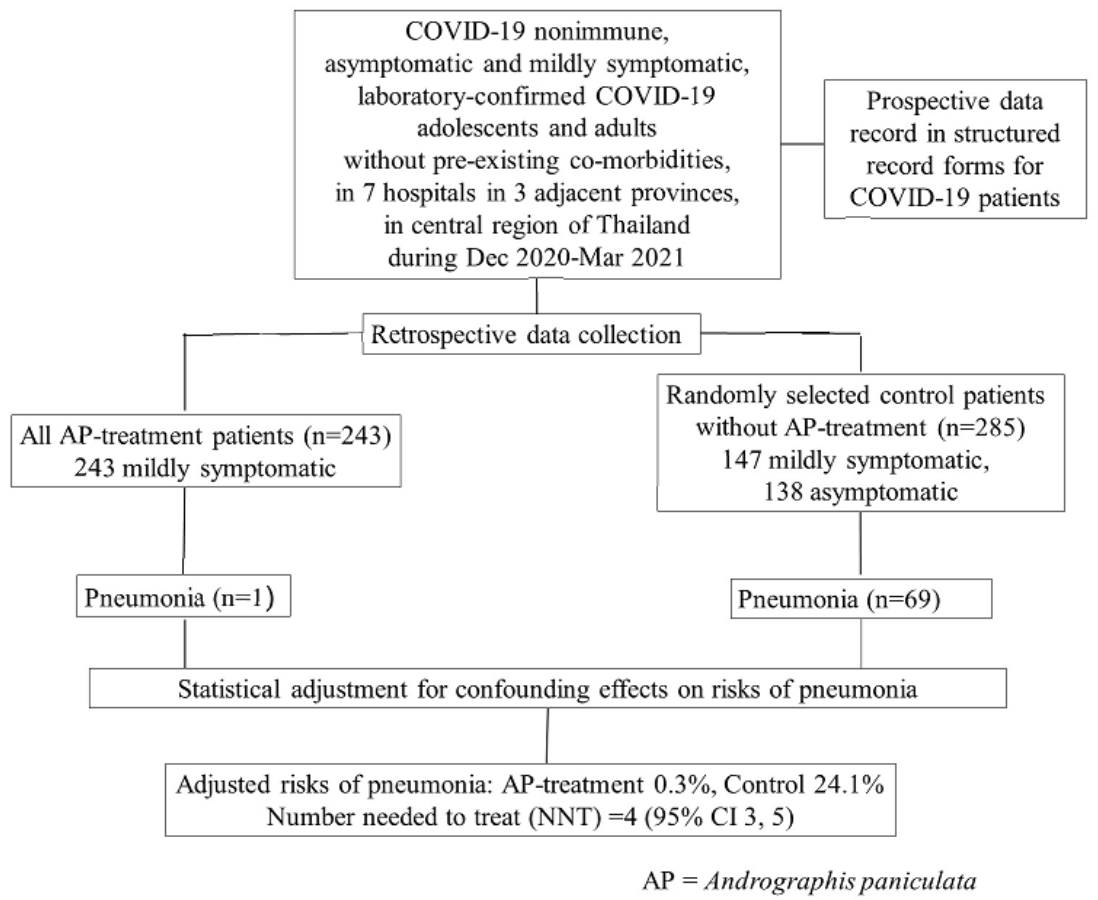
Retrospective 528 asymptomatic/mild patients in Thailand, showing lower progression to pneumonia with andrographis treatment.
|
risk of progression, 98.3% lower, RR 0.02, p < 0.001, treatment 1 of 243 (0.4%), control 69 of 285 (24.2%), NNT 4.2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Benjaponpitak et al., 31 May 2023, retrospective, Thailand, peer-reviewed, 4 authors, study period December 2020 - March 2021.
Effect of Andrographis paniculata Treatment for Nonimmune Patients with Early-Stage COVID-19 on the Prevention of Pneumonia: A Retrospective Cohort Study
Archives of Internal Medicine Research, doi:10.26502/aimr.0146
Background: Andrographis paniculata (AP) is an herbal plant that has been used to treat upper respiratory tract infections. Andrographolide is the major active component of AP that inhibits intracellular SARS-CoV-2 replication and has anti-inflammatory action. Objective: To investigate the therapeutic and adverse effects of treatment with oral AP-products in patients with early-stage COVID-19.
Methods: We performed a retrospective cohort study in COVID-19 patients with asymptomatic or mild COVID-19, admitted for isolation and treatment in seven hospitals in three adjacent provinces in central region of Thailand, during December 2020 -March 2021 epidemic when COVID-19 vaccine was not yet available and none were previously infected with SARS-CoV-2. Patient data was prospectively recorded in the structured medical record forms and retrospectively reviewed. This study included patients 15 to 60 years of age with laboratory-confirmed SARS-CoV-2 infection, but without comorbidities or pregnancy. Study AP products were capsules containing a standardised ethanol extract of AP or crude AP powder. Patients were treated for five days with either AP-extract (60 mg andrographolide, 3 times daily) or crude-AP (48 mg andrographolide, 3 times daily), only when available. All eligible patients who received AP treatment were included and control patients who did not receive AP treatment were blindly and randomly selected using a ratio of approximately 1:1. The risk of pneumonia diagnosed by chest radiography was the primary study outcome. Results: About 90% of the treatment group received the AP-extract regimen within 7 days after onset of symptoms. Pneumonia occurred in 1/243 AP treatment patients and 69/285 control patients. The risks of pneumonia after adjusting for confounding effects were 0.3% (95%CI, 0%-0.9%) and 24.3% (95%CI, 19.0%-29.7%) in the AP treatment and control groups, respectively. The number needed to treat to avoid pneumonia development in one patient was four (95% CI, 3-5). Mild abnormal symptoms suggesting adverse event of AP treatment were detected in eight patients.
Conclusion: The oral AP-extract treatment regimen is acceptably safe and associated with highly reduced rates of pneumonia in nonimmune patients with early-stage COVID-19.
Declarations
Conflict of interest: The authors declare no conflict of interest.
Ethical approval: The study was approved by the Department of Thai Traditional and Alternative Medicine, Ministry of Public Health, Thailand. Ethical approval was obtained from the Human Research Ethics Committee of Siam University (Reference Number: 2021/005). The data presented here were recorded during routine clinical practice. All data were de-identified prior to analysis and all the authors had all necessary administrative permissions to access the data.
Author Agreement All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, has not received prior publication and is not under consideration for publication elsewhere.
Consent for publication: Not applicable
References
Benjaponpitak, Sawaengtham, Thaneerat, Wanaratna, Chotsiri et al., Effect of Andrographis paniculata treatment for nonimmune patients with early-stage COVID-19 on the prevention of pneumonia: A retrospective cohort study, Archives of Internal Medicine Research
Benjaponpitak, Sawaengtham, Thaneerat, Wanaratna, Chotsiri et al., Effect of Andrographis paniculata treatment for nonimmune patients with early-stage COVID-19 on the prevention of pneumonia: A retrospective cohort study, Archives of Internal Medicine Research
Benjaponpitak, Sawaengtham, Thaneerat, Wanaratna, Chotsiri et al., Effect of Andrographis paniculata treatment for nonimmune patients with early-stage COVID-19 on the prevention of pneumonia: A retrospective cohort study, Archives of Internal Medicine Research
Benjaponpitak, Sawaengtham, Thaneerat, Wanaratna, Chotsiri et al., Effect of Andrographis paniculata treatment for nonimmune patients with early-stage COVID-19 on the prevention of pneumonia: A retrospective cohort study, Archives of Internal Medicine Research
Benjaponpitak, Sawaengtham, Thaneerat, Wanaratna, Chotsiri et al., Effect of Andrographis paniculata treatment for nonimmune patients with early-stage COVID-19 on the prevention of pneumonia: A retrospective cohort study, Archives of Internal Medicine Research
Coon, Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy, Planta Med
Enmozhi, Raja, Sebastine, Joseph, Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach, J Biomol Struct Dyn
Ervina, Pratama M R, Poerwono, Siswodihardjo, The coronavirus disease 2019 main protease inhibitor from Andrographis paniculata (Burm. f) Nees, J Adv Pharm Technol Res
Gagnier, Boon, Rochon, Moher, Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration, J Clin Epidemiol
Hiremath, Kumar, Nandan, Mantesh, In silico docking analysis revealed the potential of phytochemicals present in Phyllanthus amarus and Andrographis paniculata
Hossain, Urbi, Karuniawati, Mohiuddin, Andrographis paniculata (Burm. f.) Wall. ex Nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy, Life
Krishnasamy, Natarajan, Ramachandran, Thangaraj, Clinical outcomes among asymptomatic or mildly symptomatic COVID-19 patients in an isolation facility in Chennai, India, Am J Trop Med Hyg
Lamers, Haagmans, SARS-CoV-2 pathogenesis, Nat Rev Microbiol
Mueller, Tamura, Crowley, Degrado, Inflammatory Biomarker Trends Predict Respiratory Decline in COVID-19 Patients, Cell Rep Med
Murugan, Pandian, Jeyakanthan, Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials, J Biomol Struct Dyn
Pholphana, Panomvana, Rangkadilok, Suriyo, Andrographis paniculata: Dissolution investigation and pharmacokinetic studies of four major active diterpenoids after multiple oral dose administration in healthy Thai volunteers, J Ethnopharmacol
Phumiamorn, Sapsutthipas, Pruksakorn, Trisiriwanich, In vitro study on antiviral activity of Andrographis paniculata against COVID-19
Poolsup, Suthisisang, Prathanturarug, Asawamekin, Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials, J Clin Pharm Ther
Rajatanavin, Tuangratananon, Suphanchaimat, Tangcharoensathien, Responding to the COVID-19 second wave in Thailand by diversifying and adapting lessons from the first wave, BMJ Global Health
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Thongsri, Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod
Shi, Huang, Chen, Pi, Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage, Biochem Biophys Res Commun
Songvut, Suriyo, Panomvana, Rangkadilok, A comprehensive review on disposition kinetics and dosage of oral administration of Andrographis paniculata, an alternative herbal medicine, in co-treatment of coronavirus disease, Front Pharmacol
Tanwettiyanont, Piriyachananusorn, Sangsoi, Boonsong, Use of Andrographis paniculata (Burm.f.) Wall. ex Nees and risk of pneumonia in hospitalised patients with mild coronavirus disease 2019: A retrospective cohort study, Front. Med (
Wanaratna, Leethong, Inchai, Chueawiang, Efficacy and safety of Andrographis paniculata extract in patients with mild COVID-19: A randomized controlled trial, Arch Intern Med Res
Worakunphanich, Thavorncharoensap, Youngkong, Thadanipon, Safety of Andrographis paniculata: A systematic review and meta-analysis, Pharmacoepidemiol Drug Saf
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Yearsley, Thailand approves Asian herb Andrographis to treat COVID
Zeng, Wei, Zhou, Yuan, Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches, Phytother Res
Zhang, Lv, Zhou, Xie, Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial, Phytother Res
Zhu, Hou, Yang, Network pharmacology integrated with experimental validation revealed the antiinflammatory effects of Andrographis paniculata, Sci Rep
DOI record:
{
"DOI": "10.26502/aimr.0146",
"ISSN": [
"2688-5654"
],
"URL": "http://dx.doi.org/10.26502/aimr.0146",
"author": [
{
"affiliation": [],
"family": "Benjaponpitak",
"given": "Amporn",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sawaengtham",
"given": "Thiti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thaneerat",
"given": "Tewan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wanaratna",
"given": "Kulthanit",
"sequence": "additional"
}
],
"container-title": "Archives of Internal Medicine Research",
"container-title-short": "Arch Intern Med Res",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
7
]
],
"date-time": "2023-06-07T05:30:35Z",
"timestamp": 1686115835000
},
"deposited": {
"date-parts": [
[
2023,
6,
7
]
],
"date-time": "2023-06-07T05:30:36Z",
"timestamp": 1686115836000
},
"indexed": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T21:25:26Z",
"timestamp": 1692307526729
},
"is-referenced-by-count": 1,
"issue": "02",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "02",
"published-online": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"member": "11040",
"original-title": [],
"prefix": "10.26502",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Fortune Journals",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.fortunejournals.com/articles/effect-of-andrographis-paniculata-treatment-for-nonimmune-patients-with-earlystage-covid19-on-the-prevention-of-pneumonia-a-retros.html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Effect of Andrographis paniculata Treatment for Nonimmune Patients with Early-Stage COVID-19 on the Prevention of Pneumonia: A Retrospective Cohort Study",
"type": "journal-article",
"volume": "06"
}