Scope and Impact of Financial Conflicts of Interest in Biomedical Research
et al., JAMA, doi:10.1001/jama.289.4.454, Jan 2003
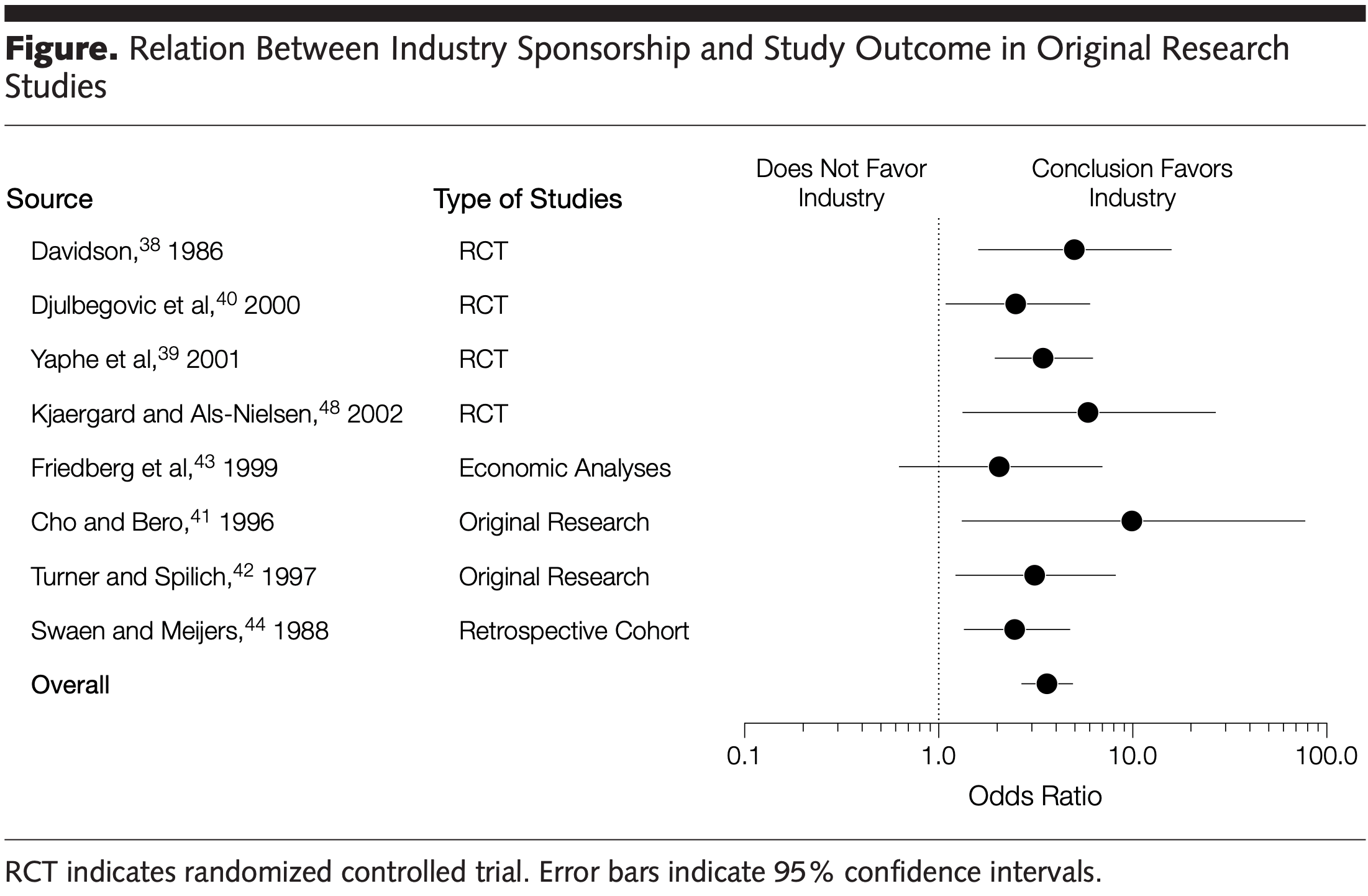
Systematic review of 37 studies examining financial conflicts of interest in biomedical research, showing that industry-sponsored studies are 3.6 times more likely to reach pro-industry conclusions compared to non-industry sponsored studies.
Bekelman et al., 22 Jan 2003, peer-reviewed, 3 authors.
Contact: cary.gross@yale.edu.
Scope and Impact of Financial Conflicts of Interest in Biomedical Research A Systematic Review
Context Despite increasing awareness about the potential impact of financial conflicts of interest on biomedical research, no comprehensive synthesis of the body of evidence relating to financial conflicts of interest has been performed.
Objective To review original, quantitative studies on the extent, impact, and management of financial conflicts of interest in biomedical research. Data Sources Studies were identified by searching MEDLINE ( January 1980-October 2002), the Web of Science citation database, references of articles, letters, commentaries, editorials, and books and by contacting experts. Study Selection All English-language studies containing original, quantitative data on financial relationships among industry, scientific investigators, and academic institutions were included. A total of 1664 citations were screened, 144 potentially eligible full articles were retrieved, and 37 studies met our inclusion criteria.
Data Extraction One investigator ( J.E.B.) extracted data from each of the 37 studies. The main outcomes were the prevalence of specific types of industry relationships, the relation between industry sponsorship and study outcome or investigator behavior, and the process for disclosure, review, and management of financial conflicts of interest.
Data Synthesis Approximately one fourth of investigators have industry affiliations, and roughly two thirds of academic institutions hold equity in start-ups that sponsor research performed at the same institutions. Eight articles, which together evaluated 1140 original studies, assessed the relation between industry sponsorship and outcome in original research. Aggregating the results of these articles showed a statistically significant association between industry sponsorship and pro-industry conclusions (pooled Mantel-Haenszel odds ratio, 3.60; 95% confidence interval, 2.63-4.91). Industry sponsorship was also associated with restrictions on publication and data sharing. The approach to managing financial conflicts varied substantially across academic institutions and peer-reviewed journals. Conclusions Financial relationships among industry, scientific investigators, and academic institutions are widespread. Conflicts of interest arising from these ties can influence biomedical research in important ways.
References
Anderson, Felson, Meenan, Secular changes in published clinical trials of second-line agents in rheumatoid arthritis, Arthritis Rheum
Asch, Jedrziewski, Christakis, Response rates to mail surveys published in medical journals, J Clin Epidemiol
Barnes, Bero, Why review articles on the health effects of passive smoking reach different conclusions, JAMA
Berk, Sacks, Assessing the quality of randomized controlled trials: quality of design is not the only relevant variable, Hepatology
Bero, Galbraith, Rennie, The publication of sponsored symposiums in medical journals, N Engl J Med
Bero, Jadad, How consumers and policymakers can use systematic reviews for decision making, Ann Intern Med
Bero, Rennie, Influences on the quality of published drug studies, Int J Technol Assess Health Care
Blumenstyk, Association announces guidelines on conflicts of interest in research involving people, Chronicle of Higher Education
Blumenthal, Campbell, Anderson, Withholding research results in academic life science: evidence from a national survey of faculty, JAMA
Blumenthal, Campbell, Causino, Louis, Participation of life-science faculty in research relationships with industry, N Engl J Med
Blumenthal, Causino, Campbell, Academicindustry research relationships in genetics: a field apart, Nat Genet
Blumenthal, Causino, Campbell, Louis, Relationships between academic institutions and industry in the life sciences: an industry survey, N Engl J Med
Blumenthal, Gluck, Louis, Universityindustry research relationships in biotechnology: implications for the university, Science
Blumenthal, Gluck, Louis, Wise, Industrial support of university research in biotechnology, Science
Blumenthal, Growing pains for new academic/ industry relationships [see comments, Health Aff
Bodenheimer, Uneasy alliance: clinical investigators and the pharmaceutical industry, N Engl J Med
Boyd, Bero, Assessing faculty financial relationships with industry, JAMA
Breslow, Day, Statistical Methods in Cancer Research
Campbell, Clarridge, Gokhale, Data withholding in academic genetics: evidence from a national survey, JAMA
Campbell, Louis, Blumenthal, Looking a gift horse in the mouth: corporate gifts supporting life sciences research, JAMA
Campbell, Weissman, Causino, Blumenthal, Data withholding in academic medicine: characteristics of faculty denied access to research results and biomaterials, Res Policy
Cho, Bero, The quality of drug studies published in symposium proceedings, Ann Intern Med
Cho, Shohara, Schissel, Policies on faculty conflicts of interest at US universities, JAMA
Cohen, Trust us to make a difference: ensuring public confidence in the integrity of clinical research, Acad Med
Cook, Mulrow, Haynes, Systematic reviews: synthesis of best evidence for clinical decisions, Ann Intern Med
Coyle, Physician-industry relations, Part 1: individual physicians, Ann Intern Med
Coyle, Physician-industry relations, Part 2: organizational issues, Ann Intern Med
Davidoff, Deangelis, Drazen, Sponsorship, authorship, and accountability, JAMA
Davidson, Source of funding and outcome of clinical trials, J Gen Intern Med
Deangelis, Fontanarosa, Flanagin, Reporting financial conflicts of interest and relationships between investigators and research sponsors, JAMA
Djulbegovic, Lacevic, Cantor, The uncertainty principle and industry-sponsored research, Lancet
Dorman, Counsell, Sandercock, Reports of randomized trials in acute stroke, 1955 to 1995: what proportions were commercially sponsored?, Stroke
Eddy, Comparing benefits and harms: the balance sheet, JAMA
Edwards, Lilford, Braunholtz, Ethical issues in the design and conduct of randomized controlled trials, Health Technol Assess
Emanuel, Steiner, Institutional conflict of interest, N Engl J Med
Friedberg, Saffran, Stinson, Evaluation of conflict of interest in economic analyses of new drugs used in oncology, JAMA
Gart, The comparison of proportions: a review of significance tests, confidence intervals and adjustments for stratification, Rev Int Statist Inst
Gelijns, Thier, Medical innovation and institutional interdependence: rethinking universityindustry connections, JAMA
Gillis, A hospital's conflict of interest: patients weren't told of stake in cancer drug, Washington Post
Healy, Campeau, Gray, Conflict of interest guidelines for a multicenter clinical trial of treatment after coronary-artery bypass-graft surgery, N Engl J Med
Hilts, Company tried to block report that its HIV vaccine failed, New York Times
Horton, Smith, Time to register randomised trials, Lancet
Hussain, Smith, Declaring financial competing interests: survey of five general medical journals, BMJ
Jadad, Moore, Carroll, Assessing the quality of reports of randomized clinical trials: is blinding necessary, Control Clin Trials
Johansen, Gotzsche, Problems in the design and reporting of trials of antifungal agents encountered during meta-analysis, JAMA
Kassirer, Angell, Financial conflicts of interest in biomedical research, N Engl J Med
Kassirer, Pseudoaccountability, Ann Intern Med
Kjaergard, Als-Nielsen, Association between competing interests and authors' conclusions: epidemiological study of randomised clinical trials published in the, BMJ
Kjaergard, Nikolova, Gluud, Randomized clinical trials in hepatology: predictors of quality, Hepatology
Knox, Adams, Djulbegovic, Reporting and dissemination of industry versus non-profit sponsored economic analyses of six novel drugs used in oncology, Ann Oncol
Korn, Conflict of interest in biomedical research, JAMA
Krimsky, Ennis, Academic-corporate ties in biotechnology: a quantitative study, Sci Technol Hum Val
Krimsky, Rothenberg, Conflict of interest policies in science and medical journals: editorial practices and author disclosures, Sci Eng Ethics
Krimsky, Rothenberg, Stott, Kyle, Scientific journals and their authors' financial interests: a pilot study, Psychother Psychosom
Levinsky, Nonfinancial conflicts of interest in research, N Engl J Med
Lo, Wolf, Berkely, Conflict-of-interest policies for investigators in clinical trials, N Engl J Med
Mantel, Haenszel, Statistical aspects of the analysis of data from retrospective studies of disease, J Natl Cancer Inst
Mccrary, Anderson, Jakovljevic, A national survey of policies on disclosure of conflicts of interest in biomedical research, N Engl J Med
Mccray, Better access to information about clinical trials, Ann Intern Med
Morin, Rakatansky, Riddick, Managing conflicts of interest in the conduct of clinical trials, JAMA
Moses, Martin, Academic relationships with industry: a new model for biomedical research, JAMA
Mulrow, Langhorne, Grimshaw, Integrating heterogeneous pieces of evidence in systematic reviews, Ann Intern Med
Press, Washburn, The kept university. Atlantic Monthly
Pressman, AUTM Licensing Survey, FY 1999: Survey Summary
Rabino, Societal and commercial issues affecting the future of biotechnology in the United States: a survey of researchers' perceptions, Naturwissenschaften
Rennie, Fair conduct and fair reporting of clinical trials, JAMA
Rennie, Thyroid storm, JAMA
Rettig, The industrialization of clinical research, Health Aff (Millwood)
Robins, Breslow, Greenland, Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models, Biometrics
Rochon, Gurwitz, Cheung, Evaluating the quality of articles published in journal supplements compared with the quality of those published in the parent journal, JAMA
Rochon, Gurwitz, Simms, A study of manufacturer-supported trials of nonsteroidal antiinflammatory drugs in the treatment of arthritis, Arch Intern Med
Rothman, Michels, The continuing unethical use of placebo controls, N Engl J Med
Schulman, Seils, Timbie, A national survey of provisions in clinical-trial agreements between medical schools and industry sponsors, N Engl J Med
Shalala, Protecting research subjects: what must be done, N Engl J Med
Slavin, Best evidence synthesis: an intelligent alternative to meta-analysis, J Clin Epidemiol
Stelfox, Chua, 'rourke, Detsky, Conflict of interest in the debate over calcium-channel antagonists, N Engl J Med
Swaen, Meijers, Influence of design characteristics on the outcome of retrospective cohort studies, Br J Ind Med
Temple, Ellenberg, Placebo-controlled trials and active-control trials in the evaluation of new treatments, Ann Intern Med
Therapy, Policy of the American Society of Gene Therapy on financial conflict of interest in clinical research
Thompson, Understanding financial conflicts of interest, N Engl J Med
Topol, Armstrong, Werf, Confronting the issues of patient safety and investigator conflict of interest in an international clinical trial of myocardial reperfusion, J Am Coll Cardiol
Turner, Spilich, Research into smoking or nicotine and human cognitive performance: does the source of funding make a difference?, Addiction
Weiss, Nelson, Teen dies undergoing experimental gene therapy, Washington Post
Wilson, Heath, Uninformed consent. The Seattle Times
Yaphe, Edman, Knishkowy, Herman, The association between funding by commercial interests and study outcome in randomized controlled drug trials, Fam Pract
DOI record:
{
"DOI": "10.1001/jama.289.4.454",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.289.4.454",
"author": [
{
"affiliation": [],
"family": "Bekelman",
"given": "Justin E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Li",
"given": "Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gross",
"given": "Cary P.",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2003,
3,
18
]
],
"date-time": "2003-03-18T15:05:58Z",
"timestamp": 1047999958000
},
"deposited": {
"date-parts": [
[
2020,
10,
29
]
],
"date-time": "2020-10-29T21:00:40Z",
"timestamp": 1604005240000
},
"indexed": {
"date-parts": [
[
2025,
12,
6
]
],
"date-time": "2025-12-06T16:52:19Z",
"timestamp": 1765039939805
},
"is-referenced-by-count": 1342,
"issue": "4",
"issued": {
"date-parts": [
[
2003,
1,
22
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2003,
1,
22
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/195843/jrv20091_454_465_1604002927.83782.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "454",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2003,
1,
22
]
]
},
"published-print": {
"date-parts": [
[
2003,
1,
22
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1001/jama.285.7.933",
"article-title": "Academic relationships with industry: a new model for biomedical research.",
"author": "Moses",
"doi-asserted-by": "publisher",
"first-page": "933",
"journal-title": "JAMA",
"key": "ref-jrv20091-1",
"volume": "285",
"year": "2001"
},
{
"DOI": "10.1056/NEJM199308193290812",
"article-title": "Understanding financial conflicts of interest.",
"author": "Thompson",
"doi-asserted-by": "publisher",
"first-page": "573",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-3",
"volume": "329",
"year": "1993"
},
{
"DOI": "10.1056/NEJMsb020853",
"article-title": "Nonfinancial conflicts of interest in research.",
"author": "Levinsky",
"doi-asserted-by": "publisher",
"first-page": "759",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-4",
"volume": "347",
"year": "2002"
},
{
"DOI": "10.1001/jama.284.17.2234",
"article-title": "Conflict of interest in biomedical research.",
"author": "Korn",
"doi-asserted-by": "publisher",
"first-page": "2234",
"journal-title": "JAMA",
"key": "ref-jrv20091-5",
"volume": "284",
"year": "2000"
},
{
"DOI": "10.1056/NEJM199308193290810",
"article-title": "Financial conflicts of interest in biomedical research.",
"author": "Kassirer",
"doi-asserted-by": "publisher",
"first-page": "570",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-6",
"volume": "329",
"year": "1993"
},
{
"DOI": "10.1001/jama.1997.03540390068038",
"article-title": "Thyroid storm.",
"author": "Rennie",
"doi-asserted-by": "publisher",
"first-page": "1238",
"journal-title": "JAMA",
"key": "ref-jrv20091-7",
"volume": "277",
"year": "1997"
},
{
"DOI": "10.1056/NEJM200005183422024",
"article-title": "Uneasy alliance: clinical investigators and the pharmaceutical industry.",
"author": "Bodenheimer",
"doi-asserted-by": "publisher",
"first-page": "1539",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-8",
"volume": "342",
"year": "2000"
},
{
"article-title": "Uninformed consent.",
"author": "Wilson",
"first-page": "1",
"journal-title": "The Seattle Times",
"key": "ref-jrv20091-9",
"year": "2001"
},
{
"article-title": "Company tried to block report that its HIV vaccine failed.",
"author": "Hilts",
"first-page": "26",
"journal-title": "New York Times",
"key": "ref-jrv20091-10",
"year": "2000"
},
{
"article-title": "A hospital's conflict of interest: patients weren't told of stake in cancer drug.",
"author": "Gillis",
"first-page": "A1",
"journal-title": "Washington Post",
"key": "ref-jrv20091-11",
"year": "2002"
},
{
"article-title": "Teen dies undergoing experimental gene therapy.",
"author": "Weiss",
"first-page": "A1",
"journal-title": "Washington Post",
"key": "ref-jrv20091-12",
"year": "1999"
},
{
"DOI": "10.1097/00001888-200102000-00028",
"article-title": "Trust us to make a difference: ensuring public confidence in the integrity of clinical research.",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "209",
"journal-title": "Acad Med",
"key": "ref-jrv20091-13",
"volume": "76",
"year": "2001"
},
{
"DOI": "10.1056/NEJM200009143431112",
"article-title": "Protecting research subjects: what must be done.",
"author": "Shalala",
"doi-asserted-by": "publisher",
"first-page": "808",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-14",
"volume": "343",
"year": "2000"
},
{
"article-title": "Association announces guidelines on conflicts of interest in research involving people.",
"author": "Blumenstyk",
"journal-title": "Chronicle of Higher Education",
"key": "ref-jrv20091-15",
"year": "2001"
},
{
"DOI": "10.7326/0003-4819-127-1-199707010-00007",
"article-title": "How consumers and policymakers can use systematic reviews for decision making.",
"author": "Bero",
"doi-asserted-by": "publisher",
"first-page": "37",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-17",
"volume": "127",
"year": "1997"
},
{
"DOI": "10.1016/0895-4356(94)00097-A",
"article-title": "Best evidence synthesis: an intelligent alternative to meta-analysis.",
"author": "Slavin",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "J Clin Epidemiol",
"key": "ref-jrv20091-18",
"volume": "48",
"year": "1995"
},
{
"DOI": "10.7326/0003-4819-126-5-199703010-00006",
"article-title": "Systematic reviews: synthesis of best evidence for clinical decisions.",
"author": "Cook",
"doi-asserted-by": "publisher",
"first-page": "376",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-19",
"volume": "126",
"year": "1997"
},
{
"article-title": "The Bayh-Dole Act of 1980, 35 USC §200-212",
"author": "Not Available",
"key": "ref-jrv20091-20",
"year": "2000"
},
{
"DOI": "10.7326/0003-4819-127-11-199712010-00008",
"article-title": "Integrating heterogeneous pieces of evidence in systematic reviews.",
"author": "Mulrow",
"doi-asserted-by": "publisher",
"first-page": "989",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-21",
"volume": "127",
"year": "1997"
},
{
"DOI": "10.1001/jama.1990.03440180103043",
"article-title": "Comparing benefits and harms: the balance sheet.",
"author": "Eddy",
"doi-asserted-by": "publisher",
"first-page": "2493",
"journal-title": "JAMA",
"key": "ref-jrv20091-22",
"volume": "263",
"year": "1990"
},
{
"DOI": "10.2307/1402171",
"article-title": "The comparison of proportions: a review of significance tests, confidence intervals and adjustments for stratification.",
"author": "Gart",
"doi-asserted-by": "publisher",
"first-page": "148",
"journal-title": "Rev Int Statist Inst",
"key": "ref-jrv20091-23",
"volume": "39",
"year": "1971"
},
{
"article-title": "Statistical aspects of the analysis of data from retrospective studies of disease.",
"author": "Mantel",
"first-page": "719",
"journal-title": "J Natl Cancer Inst",
"key": "ref-jrv20091-25",
"volume": "22",
"year": "1959"
},
{
"DOI": "10.2307/2531052",
"article-title": "Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models.",
"author": "Robins",
"doi-asserted-by": "publisher",
"first-page": "311",
"journal-title": "Biometrics",
"key": "ref-jrv20091-26",
"volume": "42",
"year": "1986"
},
{
"DOI": "10.1056/NEJM199612053352305",
"article-title": "Participation of life-science faculty in research relationships with industry.",
"author": "Blumenthal",
"doi-asserted-by": "publisher",
"first-page": "1734",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-27",
"volume": "335",
"year": "1996"
},
{
"DOI": "10.1177/016224399101600301",
"article-title": "Academic-corporate ties in biotechnology: a quantitative study.",
"author": "Krimsky",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "Sci Technol Human Values",
"key": "ref-jrv20091-28",
"volume": "16",
"year": "1991"
},
{
"DOI": "10.1159/000012281",
"article-title": "Scientific journals and their authors' financial interests: a pilot study.",
"author": "Krimsky",
"doi-asserted-by": "publisher",
"first-page": "194",
"journal-title": "Psychother Psychosom",
"key": "ref-jrv20091-29",
"volume": "67",
"year": "1998"
},
{
"DOI": "10.1126/science.3941897",
"article-title": "Industrial support of university research in biotechnology.",
"author": "Blumenthal",
"doi-asserted-by": "publisher",
"first-page": "242",
"journal-title": "Science",
"key": "ref-jrv20091-30",
"volume": "231",
"year": "1986"
},
{
"DOI": "10.1056/NEJM199602083340606",
"article-title": "Relationships between academic institutions and industry in the life sciences: an industry survey.",
"author": "Blumenthal",
"doi-asserted-by": "publisher",
"first-page": "368",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-31",
"volume": "334",
"year": "1996"
},
{
"DOI": "10.1126/science.3715452",
"article-title": "University-industry research relationships in biotechnology: implications for the university.",
"author": "Blumenthal",
"doi-asserted-by": "publisher",
"first-page": "1361",
"journal-title": "Science",
"key": "ref-jrv20091-33",
"volume": "232",
"year": "1986"
},
{
"DOI": "10.1038/ng0597-104",
"article-title": "Academic-industry research relationships in genetics: a field apart.",
"author": "Blumenthal",
"doi-asserted-by": "publisher",
"first-page": "104",
"journal-title": "Nat Genet",
"key": "ref-jrv20091-34",
"volume": "16",
"year": "1997"
},
{
"DOI": "10.1001/jama.279.13.995",
"article-title": "Looking a gift horse in the mouth: corporate gifts supporting life sciences research.",
"author": "Campbell",
"doi-asserted-by": "publisher",
"first-page": "995",
"journal-title": "JAMA",
"key": "ref-jrv20091-35",
"volume": "279",
"year": "1998"
},
{
"DOI": "10.1001/jama.284.17.2209",
"article-title": "Assessing faculty financial relationships with industry.",
"author": "Boyd",
"doi-asserted-by": "publisher",
"first-page": "2209",
"journal-title": "JAMA",
"key": "ref-jrv20091-36",
"volume": "284",
"year": "2000"
},
{
"DOI": "10.1016/S0895-4356(97)00126-1",
"article-title": "Response rates to mail surveys published in medical journals.",
"author": "Asch",
"doi-asserted-by": "publisher",
"first-page": "1129",
"journal-title": "J Clin Epidemiol",
"key": "ref-jrv20091-37",
"volume": "50",
"year": "1997"
},
{
"DOI": "10.1007/BF02602327",
"article-title": "Source of funding and outcome of clinical trials.",
"author": "Davidson",
"doi-asserted-by": "publisher",
"first-page": "155",
"journal-title": "J Gen Intern Med",
"key": "ref-jrv20091-38",
"volume": "1",
"year": "1986"
},
{
"DOI": "10.1093/fampra/18.6.565",
"article-title": "The association between funding by commercial interests and study outcome in randomized controlled drug trials.",
"author": "Yaphe",
"doi-asserted-by": "publisher",
"first-page": "565",
"journal-title": "Fam Pract",
"key": "ref-jrv20091-39",
"volume": "18",
"year": "2001"
},
{
"DOI": "10.1016/S0140-6736(00)02605-2",
"article-title": "The uncertainty principle and industry-sponsored research.",
"author": "Djulbegovic",
"doi-asserted-by": "publisher",
"first-page": "635",
"journal-title": "Lancet",
"key": "ref-jrv20091-40",
"volume": "356",
"year": "2000"
},
{
"DOI": "10.7326/0003-4819-124-5-199603010-00004",
"article-title": "The quality of drug studies published in symposium proceedings.",
"author": "Cho",
"doi-asserted-by": "publisher",
"first-page": "485",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-41",
"volume": "124",
"year": "1996"
},
{
"DOI": "10.1111/j.1360-0443.1997.tb02863.x",
"article-title": "Research into smoking or nicotine and human cognitive performance: does the source of funding make a difference?",
"author": "Turner",
"doi-asserted-by": "publisher",
"first-page": "1423",
"journal-title": "Addiction",
"key": "ref-jrv20091-42",
"volume": "92",
"year": "1997"
},
{
"DOI": "10.1001/jama.282.15.1453",
"article-title": "Evaluation of conflict of interest in economic analyses of new drugs used in oncology.",
"author": "Friedberg",
"doi-asserted-by": "publisher",
"first-page": "1453",
"journal-title": "JAMA",
"key": "ref-jrv20091-43",
"volume": "282",
"year": "1999"
},
{
"article-title": "Influence of design characteristics on the outcome of retrospective cohort studies.",
"author": "Swaen",
"first-page": "624",
"journal-title": "Br J Ind Med",
"key": "ref-jrv20091-44",
"volume": "45",
"year": "1988"
},
{
"DOI": "10.1001/archinte.1994.00420020059007",
"article-title": "A study of manufacturer-supported trials of nonsteroidal anti-inflammatory drugs in the treatment of arthritis.",
"author": "Rochon",
"doi-asserted-by": "publisher",
"first-page": "157",
"journal-title": "Arch Intern Med",
"key": "ref-jrv20091-45",
"volume": "154",
"year": "1994"
},
{
"DOI": "10.1056/NEJM199801083380206",
"article-title": "Conflict of interest in the debate over calcium-channel antagonists.",
"author": "Stelfox",
"doi-asserted-by": "publisher",
"first-page": "101",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-46",
"volume": "338",
"year": "1998"
},
{
"DOI": "10.1001/jama.279.19.1566",
"article-title": "Why review articles on the health effects of passive smoking reach different conclusions.",
"author": "Barnes",
"doi-asserted-by": "publisher",
"first-page": "1566",
"journal-title": "JAMA",
"key": "ref-jrv20091-47",
"volume": "279",
"year": "1998"
},
{
"DOI": "10.1136/bmj.325.7358.249",
"article-title": "Association between competing interests and authors' conclusions: epidemiological study of randomised clinical trials published in the BMJ.",
"author": "Kjaergard",
"doi-asserted-by": "publisher",
"first-page": "249",
"journal-title": "BMJ",
"key": "ref-jrv20091-48",
"volume": "325",
"year": "2002"
},
{
"DOI": "10.1001/jama.282.18.1752",
"article-title": "Problems in the design and reporting of trials of antifungal agents encountered during meta-analysis.",
"author": "Johansen",
"doi-asserted-by": "publisher",
"first-page": "1752",
"journal-title": "JAMA",
"key": "ref-jrv20091-49",
"volume": "282",
"year": "1999"
},
{
"DOI": "10.1002/(ISSN)1527-3350",
"article-title": "Randomized clinical trials in hepatology: predictors of quality.",
"author": "Kjaergard",
"doi-asserted-by": "publisher",
"first-page": "1134",
"journal-title": "Hepatology",
"key": "ref-jrv20091-50",
"volume": "30",
"year": "1999"
},
{
"DOI": "10.1002/(ISSN)1529-0131",
"article-title": "Secular changes in published clinical trials of second-line agents in rheumatoid arthritis.",
"author": "Anderson",
"doi-asserted-by": "publisher",
"first-page": "1304",
"journal-title": "Arthritis Rheum",
"key": "ref-jrv20091-51",
"volume": "34",
"year": "1991"
},
{
"DOI": "10.1023/A:1008309817708",
"article-title": "Reporting and dissemination of industry versus non-profit sponsored economic analyses of six novel drugs used in oncology.",
"author": "Knox",
"doi-asserted-by": "publisher",
"first-page": "1591",
"journal-title": "Ann Oncol",
"key": "ref-jrv20091-52",
"volume": "11",
"year": "2000"
},
{
"DOI": "10.1001/jama.1994.03520020034009",
"article-title": "Evaluating the quality of articles published in journal supplements compared with the quality of those published in the parent journal.",
"author": "Rochon",
"doi-asserted-by": "publisher",
"first-page": "108",
"journal-title": "JAMA",
"key": "ref-jrv20091-53",
"volume": "272",
"year": "1994"
},
{
"DOI": "10.1056/NEJM199210153271606",
"article-title": "The publication of sponsored symposiums in medical journals.",
"author": "Bero",
"doi-asserted-by": "publisher",
"first-page": "1135",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-54",
"volume": "327",
"year": "1992"
},
{
"DOI": "10.1001/jama.1997.03540390054035",
"article-title": "Withholding research results in academic life science: evidence from a national survey of faculty.",
"author": "Blumenthal",
"doi-asserted-by": "publisher",
"first-page": "1224",
"journal-title": "JAMA",
"key": "ref-jrv20091-55",
"volume": "277",
"year": "1997"
},
{
"DOI": "10.1016/S0048-7333(99)00068-2",
"article-title": "Data withholding in academic medicine: characteristics of faculty denied access to research results and biomaterials.",
"author": "Campbell",
"doi-asserted-by": "publisher",
"first-page": "303",
"journal-title": "Res Policy",
"key": "ref-jrv20091-56",
"volume": "29",
"year": "2000"
},
{
"DOI": "10.1001/jama.287.4.473",
"article-title": "Data withholding in academic genetics: evidence from a national survey.",
"author": "Campbell",
"doi-asserted-by": "publisher",
"first-page": "473",
"journal-title": "JAMA",
"key": "ref-jrv20091-57",
"volume": "287",
"year": "2002"
},
{
"DOI": "10.1007/s001140050464",
"article-title": "Societal and commercial issues affecting the future of biotechnology in the United States: a survey of researchers' perceptions.",
"author": "Rabino",
"doi-asserted-by": "publisher",
"first-page": "109",
"journal-title": "Naturwissenschaften",
"key": "ref-jrv20091-58",
"volume": "85",
"year": "1998"
},
{
"DOI": "10.1056/NEJM200011303432207",
"article-title": "A national survey of policies on disclosure of conflicts of interest in biomedical research.",
"author": "McCrary",
"doi-asserted-by": "publisher",
"first-page": "1621",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-59",
"volume": "343",
"year": "2000"
},
{
"DOI": "10.1001/jama.284.17.2203",
"article-title": "Policies on faculty conflicts of interest at US universities.",
"author": "Cho",
"doi-asserted-by": "publisher",
"first-page": "2203",
"journal-title": "JAMA",
"key": "ref-jrv20091-60",
"volume": "284",
"year": "2000"
},
{
"DOI": "10.1056/NEJM200011303432206",
"article-title": "Conflict-of-interest policies for investigators in clinical trials.",
"author": "Lo",
"doi-asserted-by": "publisher",
"first-page": "1616",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-61",
"volume": "343",
"year": "2000"
},
{
"DOI": "10.1007/s11948-001-0041-7",
"article-title": "Conflict of interest policies in science and medical journals: editorial practices and author disclosures.",
"author": "Krimsky",
"doi-asserted-by": "publisher",
"first-page": "205",
"journal-title": "Sci Eng Ethics",
"key": "ref-jrv20091-62",
"volume": "7",
"year": "2001"
},
{
"DOI": "10.1161/01.STR.30.10.1995",
"article-title": "Reports of randomized trials in acute stroke, 1955 to 1995: what proportions were commercially sponsored?",
"author": "Dorman",
"doi-asserted-by": "publisher",
"first-page": "1995",
"journal-title": "Stroke",
"key": "ref-jrv20091-63",
"volume": "30",
"year": "1999"
},
{
"DOI": "10.1136/bmj.323.7307.263",
"article-title": "Declaring financial competing interests: survey of five general medical journals.",
"author": "Hussain",
"doi-asserted-by": "publisher",
"first-page": "263",
"journal-title": "BMJ",
"key": "ref-jrv20091-64",
"volume": "323",
"year": "2001"
},
{
"DOI": "10.1056/NEJMsa020349",
"article-title": "A national survey of provisions in clinical-trial agreements between medical schools and industry sponsors.",
"author": "Schulman",
"doi-asserted-by": "publisher",
"first-page": "1335",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-65",
"volume": "347",
"year": "2002"
},
{
"DOI": "10.1017/S0266462300009582",
"article-title": "Influences on the quality of published drug studies.",
"author": "Bero",
"doi-asserted-by": "publisher",
"first-page": "209",
"journal-title": "Int J Technol Assess Health Care",
"key": "ref-jrv20091-66",
"volume": "12",
"year": "1996"
},
{
"DOI": "10.1056/NEJM199408113310611",
"article-title": "The continuing unethical use of placebo controls.",
"author": "Rothman",
"doi-asserted-by": "publisher",
"first-page": "394",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-67",
"volume": "331",
"year": "1994"
},
{
"DOI": "10.7326/0003-4819-133-6-200009190-00014",
"article-title": "Placebo-controlled trials and active-control trials in the evaluation of new treatments.",
"author": "Temple",
"doi-asserted-by": "publisher",
"first-page": "455",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-68",
"volume": "133",
"year": "2000"
},
{
"article-title": "Adequate and Well-controlled Studies, 21 CFR §314.126",
"author": "Not Available",
"key": "ref-jrv20091-69",
"year": "2001"
},
{
"DOI": "10.3310/hta2150",
"article-title": "Ethical issues in the design and conduct of randomized controlled trials.",
"author": "Edwards",
"doi-asserted-by": "crossref",
"journal-title": "Health Technol Assess",
"key": "ref-jrv20091-71",
"volume": "2",
"year": "1998"
},
{
"DOI": "10.1001/jama.282.18.1766",
"article-title": "Fair conduct and fair reporting of clinical trials.",
"author": "Rennie",
"doi-asserted-by": "publisher",
"first-page": "1766",
"journal-title": "JAMA",
"key": "ref-jrv20091-72",
"volume": "282",
"year": "1999"
},
{
"DOI": "10.1002/(ISSN)1527-3350",
"article-title": "Assessing the quality of randomized controlled trials: quality of design is not the only relevant variable.",
"author": "Berk",
"doi-asserted-by": "publisher",
"first-page": "1332",
"journal-title": "Hepatology",
"key": "ref-jrv20091-73",
"volume": "30",
"year": "1999"
},
{
"DOI": "10.1016/0197-2456(95)00134-4",
"article-title": "Assessing the quality of reports of randomized clinical trials: is blinding necessary.",
"author": "Jadad",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Control Clin Trials",
"key": "ref-jrv20091-74",
"volume": "17",
"year": "1996"
},
{
"DOI": "10.1056/NEJM199501263320412",
"article-title": "Institutional conflict of interest.",
"author": "Emanuel",
"doi-asserted-by": "publisher",
"first-page": "262",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-75",
"volume": "332",
"year": "1995"
},
{
"DOI": "10.1377/hlthaff.13.3.176",
"article-title": "Growing pains for new academic/industry relationships [see comments].",
"author": "Blumenthal",
"doi-asserted-by": "publisher",
"first-page": "176",
"journal-title": "Health Aff (Millwood)",
"key": "ref-jrv20091-76",
"volume": "13",
"year": "1994"
},
{
"DOI": "10.1001/jama.287.1.72",
"article-title": "Medical innovation and institutional interdependence: rethinking university-industry connections.",
"author": "Gelijns",
"doi-asserted-by": "publisher",
"first-page": "72",
"journal-title": "JAMA",
"key": "ref-jrv20091-77",
"volume": "287",
"year": "2002"
},
{
"DOI": "10.1056/NEJM198904063201432",
"article-title": "Conflict of interest guidelines for a multicenter clinical trial of treatment after coronary-artery bypass-graft surgery.",
"author": "Healy",
"doi-asserted-by": "publisher",
"first-page": "949",
"journal-title": "N Engl J Med",
"key": "ref-jrv20091-79",
"volume": "320",
"year": "1989"
},
{
"DOI": "10.1016/0735-1097(92)90312-B",
"article-title": "Confronting the issues of patient safety and investigator conflict of interest in an international clinical trial of myocardial reperfusion.",
"author": "Topol",
"doi-asserted-by": "publisher",
"first-page": "1123",
"journal-title": "J Am Coll Cardiol",
"key": "ref-jrv20091-80",
"volume": "19",
"year": "1992"
},
{
"DOI": "10.7326/0003-4819-136-5-200203050-00014",
"article-title": "Physician-industry relations, Part 1: individual physicians.",
"author": "Coyle",
"doi-asserted-by": "publisher",
"first-page": "396",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-82",
"volume": "136",
"year": "2002"
},
{
"DOI": "10.7326/0003-4819-136-5-200203050-00015",
"article-title": "Physician-industry relations, Part 2: organizational issues.",
"author": "Coyle",
"doi-asserted-by": "publisher",
"first-page": "403",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-83",
"volume": "136",
"year": "2002"
},
{
"DOI": "10.1001/jama.287.1.78",
"article-title": "Managing conflicts of interest in the conduct of clinical trials.",
"author": "Morin",
"doi-asserted-by": "publisher",
"first-page": "78",
"journal-title": "JAMA",
"key": "ref-jrv20091-84",
"volume": "287",
"year": "2002"
},
{
"DOI": "10.1001/jama.286.1.89",
"article-title": "Reporting financial conflicts of interest and relationships between investigators and research sponsors.",
"author": "DeAngelis",
"doi-asserted-by": "publisher",
"first-page": "89",
"journal-title": "JAMA",
"key": "ref-jrv20091-86",
"volume": "286",
"year": "2001"
},
{
"DOI": "10.1001/jama.286.10.1232",
"article-title": "Sponsorship, authorship, and accountability.",
"author": "Davidoff",
"doi-asserted-by": "publisher",
"first-page": "1232",
"journal-title": "JAMA",
"key": "ref-jrv20091-87",
"volume": "286",
"year": "2001"
},
{
"DOI": "10.1377/hlthaff.19.2.129",
"article-title": "The industrialization of clinical research.",
"author": "Rettig",
"doi-asserted-by": "publisher",
"first-page": "129",
"journal-title": "Health Aff (Millwood)",
"key": "ref-jrv20091-88",
"volume": "19",
"year": "2000"
},
{
"article-title": "The kept university.",
"author": "Press",
"first-page": "39",
"journal-title": "Atlantic Monthly",
"key": "ref-jrv20091-89",
"year": "2000"
},
{
"DOI": "10.7326/0003-4819-134-7-200104030-00012",
"article-title": "Pseudoaccountability.",
"author": "Kassirer",
"doi-asserted-by": "publisher",
"first-page": "587",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-90",
"volume": "134",
"year": "2001"
},
{
"DOI": "10.1016/S0140-6736(99)00328-1",
"article-title": "Time to register randomised trials.",
"author": "Horton",
"doi-asserted-by": "publisher",
"first-page": "1138",
"journal-title": "Lancet",
"key": "ref-jrv20091-91",
"volume": "354",
"year": "1999"
},
{
"DOI": "10.7326/0003-4819-133-8-200010170-00013",
"article-title": "Better access to information about clinical trials.",
"author": "McCray",
"doi-asserted-by": "publisher",
"first-page": "609",
"journal-title": "Ann Intern Med",
"key": "ref-jrv20091-92",
"volume": "133",
"year": "2000"
},
{
"author": "Not Available",
"key": "ref-jrv20091-2",
"volume-title": "National Institutes of Health Extramural Data and Trends",
"year": "2000"
},
{
"author": "Task Force on Financial Conflicts of Interest in Clinical Research",
"key": "ref-jrv20091-16",
"volume-title": "Protecting Subjects, Preserving Trust, Promoting Progress: Policy and Guidelines for the Oversight of Individual Financial Interests in Human Subjects Research",
"year": "2001"
},
{
"author": "Breslow",
"key": "ref-jrv20091-24",
"volume-title": "Statistical Methods in Cancer Research",
"year": "1987"
},
{
"author": "Pressman",
"key": "ref-jrv20091-32",
"volume-title": "AUTM Licensing Survey, FY 1999: Survey Summary",
"year": "2000"
},
{
"author": "Not Available",
"key": "ref-jrv20091-70",
"volume-title": "Guidance for Industry: Choice of Control Group and Related Issues in Clinical Trials",
"year": "2001"
},
{
"author": "Not Available",
"key": "ref-jrv20091-81",
"volume-title": "Report on Individual and Institutional Financial Conflict of Interest",
"year": "2001"
},
{
"author": "Task Force on Financial Conflicts of Interest in Clinical Research",
"key": "ref-jrv20091-85",
"volume-title": "Protecting Subjects, Preserving Trust, Promoting Progress II: Principles and Recommendations for Oversight of an Institution's Financial Interests in Human Subjects Research",
"year": "2002"
},
{
"key": "ref-jrv20091-78",
"unstructured": "American Society of Gene Therapy. Policy of the American Society of Gene Therapy on financial conflict of interest in clinical research [adopted April 5, 2000]. Available at: http://www.asgt.org/policy/index.html. Accessed June 5, 2002."
}
],
"reference-count": 92,
"references-count": 92,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/195843"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"A Systematic Review"
],
"title": "Scope and Impact of Financial Conflicts of Interest in Biomedical Research",
"type": "journal-article",
"volume": "289"
}