Effects of nebulized hypertonic saline on inflammatory mediators in patients with severe COVID-19 pneumonia: A double-blinded randomized controlled trial
et al., Science Progress, doi:10.1177/00368504231203130, Oct 2023
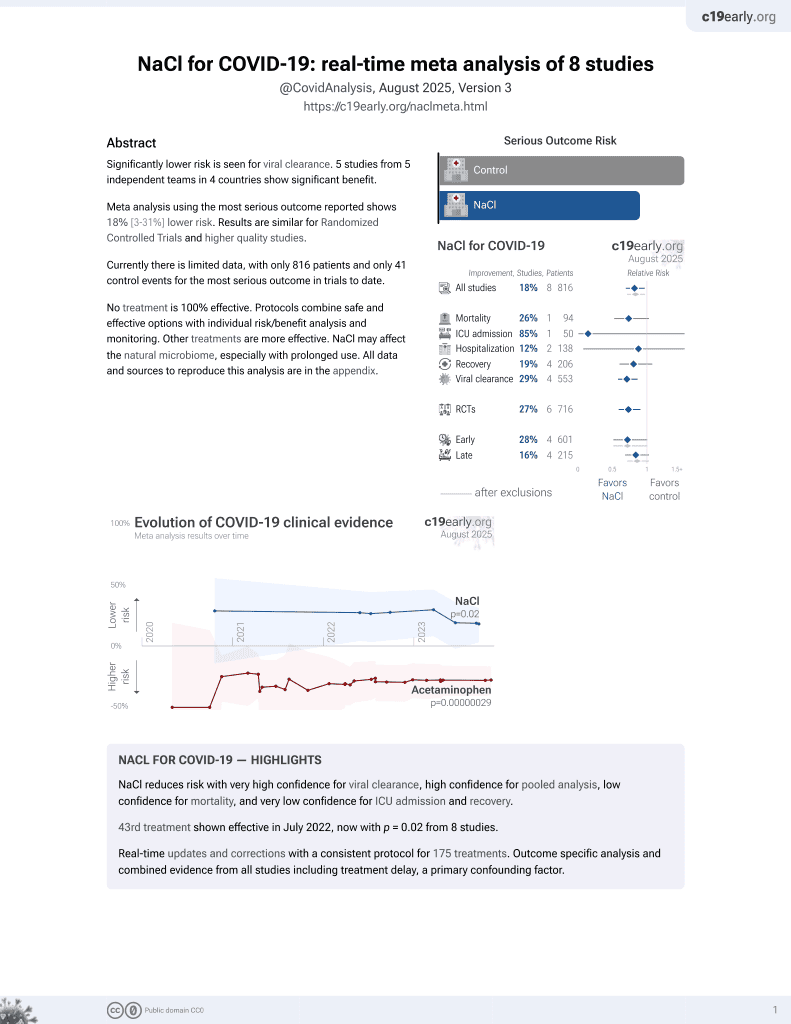
NaCl for COVID-19
44th treatment shown to reduce risk in
July 2022, now with p = 0.0028 from 9 studies.
Lower risk for progression and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 60 severe COVID-19 pneumonia patients showing significant reduction in inflammatory markers (TNF-α, IL-6) and improved oxygenation with nebulized 5% hypertonic saline compared to distilled water. 50% vs. 36% were on noninvasive ventilation at baseline in the treatment group. Data is not clear in this study: Table 3 shows baseline hospital stays average 40 and 39.3 days in each group, compared with 37 and 38.6 days after a 5-day intervention. The baseline table reports comorbidity percentages against the total number of patients with each condition rather than the 30 patients per arm.
Beigmohammadi et al., 3 Oct 2023, Double Blind Randomized Controlled Trial, Iran, peer-reviewed, mean age 50.9, 10 authors, study period December 2021 - February 2022, this trial compares with another treatment - results may be better when compared to placebo.
Contact: elhamnazar@yahoo.com.
Effects of nebulized hypertonic saline on inflammatory mediators in patients with severe COVID-19 pneumonia: A double-blinded randomized controlled trial
Science Progress, doi:10.1177/00368504231203130
Introduction: An exaggerated immune response is considered the most important aspect of COVID-19 pathogenesis. Hypertonic saline (HS) has shown promise in combating inflammation in several respiratory diseases. We investigated the effects of nebulized HS on clinical symptoms and inflammatory status in patients with severe novel coronavirus infection (COVID-19) pneumonia.
Declaration of conflicting interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/ or publication of this article.
Ethics approval This trial was approved by the Ethical Committee of the Tehran University of Medical Sciences (TR.TUMS.IKHC.REC.1400.016).
Research registration number The study was registered at www.irct.ir (registration number: IRCT20120216009045N4).
Author biographies Mohammad-Taghi Beigmohammadi is a professor of anesthesiology and intensive care and is a fellowship of critical care medicine. He is head of the ICU at Imam Khomeini Complex Hospital. Laya Amoozadeh has a Ph.D. in physiotherapy and works and research in the school of rehabilitation at Tehran University of Medical Sciences. Nikoosadat Naghibi is an assistant professor of anesthesiology at Abadan University of Medical Sciences and works in the ICU. Babak Eslami is an assistant professor of anesthesiology and research on pulmonary diseases.
Samrand Fattah Ghazi is an assistant professor of anesthesiology and research on viral pulmonary infections and treatment. Mohammad Javaherian has a Ph.D. in physiotherapy and works and research in the school of rehabilitation at Tehran University of Medical Sciences. Mohammad-Amin Khajeh-Azad is a general practitioner and does statistical analysis as a consultant. Bahram Tabatabaei has a Ph.D. in physiotherapy and works and research in the school of rehabilitation at Tehran University of Medical..
References
Aitken, Greene, Tonelli, Analysis of sequential aliquots of hypertonic saline solution-induced sputum from clinically stable patients with cystic fibrosis, Chest
Angle, Hoyt, Coimbra, Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock, Shock
Bahrami, Zimmermann, Szelenyi, Small-volume fluid resuscitation with hypertonic saline prevents inflammation but not mortality in a rat model of hemorrhagic shock, Shock
Banerjee, Moore, Mclaughlin, Hyperosmolarity attenuates TNF-alphamediated proinflammatory activation of human pulmonary microvascular endothelial cells, 12 Science Progress
Bergsson, Reeves, Mcnally, LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline, J Immunol
Carpagnano, Barbaro, Cagnazzo, Use of exhaled breath condensate in the study of airway inflammation after hypertonic saline solution challenge, Chest
Carro, Ma, Nebulized hypertonic saline in non-cystic fibrosis bronchiectasis: a comprehensive review, Ther Adv Respir Dis
Cuschieri, Gourlay, Garcia, Hypertonic preconditioning inhibits macrophage responsiveness to endotoxin, J Immunol
De Boer, Waterlander, Kuijper, Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate, Int J Behav Nutr Phys Act
Dmello, Nayak, Matuschak, Stratified assessment of the role of inhaled hypertonic saline in reducing cystic fibrosis pulmonary exacerbations: a retrospective analysis, BMJ Open
Elkins, Robinson, Rose, A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis, N Engl J Med
Karakozis, Hinds, Cook, The effects of interleukin-10 in hemorrhagic shock, J Surg Res
Kuzik, Sa, Kent, Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants, J Pediatr
Lee, Data transformation: a focus on the interpretation, Korean J Anesthesiol
Mcelvaney, Mcevoy, Boland, A randomized, double-blind, placebo-controlled trial of intravenous alpha-1 antitrypsin for ARDS secondary to COVID-19, Med
Mitra, Schiller, Anderson, Hypertonic saline attenuates the cytokine-induced pro-inflammatory signature in primary human lung epithelia, PLoS One
Moore, De Waal Malefyt, Coffman, Interleukin-10 and the interleukin-10 receptor, Annu Rev Immunol
Nydam, Moore, Mcintyre, Hypertonic saline attenuates TNF-alphainduced NF-kappaB activation in pulmonary epithelial cells, Shock
Of, Mohame, Patient clinical pathway COVID-19
Organization, Living guidance for clinical management of COVID-19: living guidance
Powers, Zurawska, Szaszi, Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion, Surgery
Reeves, Molloy, Pohl, Hypertonic saline in the treatment of pulmonary disease in cystic fibrosis, ScientificWorldJournal
Standiford, Anti-inflammatory cytokines and cytokine antagonists, Curr Pharm Des
Wohlauer, Moore, Silliman, Nebulized hypertonic saline attenuates acute lung injury following trauma and hemorrhagic shock via inhibition of matrix metalloproteinase-13, Crit Care Med
Wright, Gamboni, Moore, Hyperosmolarity invokes distinct anti-inflammatory mechanisms in pulmonary epithelial cells: evidence from signaling and transcription layers, PLoS One
DOI record:
{
"DOI": "10.1177/00368504231203130",
"ISSN": [
"0036-8504",
"2047-7163"
],
"URL": "http://dx.doi.org/10.1177/00368504231203130",
"abstract": "<jats:sec><jats:title>Introduction:</jats:title><jats:p> An exaggerated immune response is considered the most important aspect of COVID-19 pathogenesis. Hypertonic saline (HS) has shown promise in combating inflammation in several respiratory diseases. We investigated the effects of nebulized HS on clinical symptoms and inflammatory status in patients with severe novel coronavirus infection (COVID-19) pneumonia. </jats:p></jats:sec><jats:sec><jats:title>Materials and Methods:</jats:title><jats:p> We randomly assigned 60 adults admitted to the intensive care unit (ICU) due to severe COVID-19 pneumonia to the experimental (received nebulized 5% saline) and control (received nebulized distilled water) groups. All interventions were applied 4 times daily for 5 days. The levels of tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and other clinical factors from venous blood were evaluated before and after intervention application. Mortality rate, intubation rate, and durations of ICU and hospital stay were also compared between groups. </jats:p></jats:sec><jats:sec><jats:title>Results:</jats:title><jats:p> The levels of TNF-α (MD: −21.35 [−32.29, −10.40], P = 0.000) and IL-6 (−9.94 [−18.86, −1.02], P = 0.003) were lower in the experimental group compared to the control group after applying the interventions. The levels of white blood cell count, PO<jats:sub>2</jats:sub>, and serum sodium were also statistically significant differences between groups. However, we did not observe significant differences in terms of hospitalization durations and mortality rates. </jats:p></jats:sec><jats:sec><jats:title>Conclusion:</jats:title><jats:p> Nebulization of HS in patients with severe COVID-19 pneumonia appears to be effective in reducing inflammation, but does not appear to affect intubation rates, mortality, hospitalization, or length of stay in ICU. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/00368504231203130"
],
"author": [
{
"affiliation": [
{
"name": "Department of Intensive Care Unit, Imam Khomeini Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Beigmohammadi",
"given": "Mohammad-Taghi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Unit, Imam Khomeini Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Amoozadeh",
"given": "Laya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Unit, Imam Khomeini Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Naghibi",
"given": "Nikoosadat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Unit, Imam Khomeini Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Eslami",
"given": "Babak",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Unit, Imam Khomeini Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Fattah Ghazi",
"given": "Samrand",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Physiotherapy, School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Javaherian",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "General Practitioner, Iran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Khajeh-Azad",
"given": "Mohammad-Amin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Physiotherapy, School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Tabatabaei",
"given": "Bahram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, School of Medicine, Imam Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"family": "Abdollahi",
"given": "Alireza",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6182-3893",
"affiliation": [
{
"name": "Department of Pathology, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran"
}
],
"authenticated-orcid": false,
"family": "Nazar",
"given": "Elham",
"sequence": "additional"
}
],
"container-title": "Science Progress",
"container-title-short": "Science Progress",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2023,
10,
3
]
],
"date-time": "2023-10-03T11:46:23Z",
"timestamp": 1696333583000
},
"deposited": {
"date-parts": [
[
2025,
3,
3
]
],
"date-time": "2025-03-03T12:45:08Z",
"timestamp": 1741005908000
},
"indexed": {
"date-parts": [
[
2025,
3,
4
]
],
"date-time": "2025-03-04T05:39:10Z",
"timestamp": 1741066750205,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
10
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
1
]
],
"date-time": "2023-10-01T00:00:00Z",
"timestamp": 1696118400000
}
}
],
"link": [
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/00368504231203130",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/full-xml/10.1177/00368504231203130",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/00368504231203130",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"prefix": "10.1177",
"published": {
"date-parts": [
[
2023,
10
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
3
]
]
},
"published-print": {
"date-parts": [
[
2023,
10
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"author": "Organization WH",
"key": "bibr1-00368504231203130",
"volume-title": "Living guidance for clinical management of COVID-19: living guidance, 23 November 2021",
"year": "2021"
},
{
"key": "bibr2-00368504231203130",
"unstructured": "Deputy of Treatment MoHaME. Patient clinical pathway COVID-19. 2020."
},
{
"DOI": "10.1016/j.medj.2022.03.001",
"doi-asserted-by": "publisher",
"key": "bibr3-00368504231203130"
},
{
"DOI": "10.1100/2012/465230",
"doi-asserted-by": "publisher",
"key": "bibr4-00368504231203130"
},
{
"DOI": "10.1056/NEJMoa043900",
"doi-asserted-by": "publisher",
"key": "bibr5-00368504231203130"
},
{
"DOI": "10.1136/bmjopen-2010-000019",
"doi-asserted-by": "publisher",
"key": "bibr6-00368504231203130"
},
{
"DOI": "10.1177/1753466619866102",
"doi-asserted-by": "publisher",
"key": "bibr7-00368504231203130"
},
{
"DOI": "10.1016/j.jpeds.2007.04.010",
"doi-asserted-by": "publisher",
"key": "bibr8-00368504231203130"
},
{
"DOI": "10.1378/chest.123.3.792",
"doi-asserted-by": "publisher",
"key": "bibr9-00368504231203130"
},
{
"DOI": "10.4049/jimmunol.0803959",
"doi-asserted-by": "publisher",
"key": "bibr10-00368504231203130"
},
{
"DOI": "10.4097/kja.20137",
"doi-asserted-by": "publisher",
"key": "bibr11-00368504231203130"
},
{
"DOI": "10.1186/s12966-015-0162-z",
"doi-asserted-by": "publisher",
"key": "bibr12-00368504231203130"
},
{
"DOI": "10.1146/annurev.immunol.19.1.683",
"doi-asserted-by": "publisher",
"key": "bibr13-00368504231203130"
},
{
"DOI": "10.2174/1381612003400533",
"doi-asserted-by": "publisher",
"key": "bibr14-00368504231203130"
},
{
"DOI": "10.1006/jsre.2000.5860",
"doi-asserted-by": "publisher",
"key": "bibr15-00368504231203130"
},
{
"DOI": "10.1097/SHK.0b013e31818ec47d",
"doi-asserted-by": "publisher",
"key": "bibr16-00368504231203130"
},
{
"DOI": "10.1371/journal.pone.0114129",
"doi-asserted-by": "publisher",
"key": "bibr17-00368504231203130"
},
{
"DOI": "10.1016/j.surg.2004.05.051",
"doi-asserted-by": "publisher",
"key": "bibr18-00368504231203130"
},
{
"DOI": "10.4049/jimmunol.168.3.1389",
"doi-asserted-by": "publisher",
"key": "bibr19-00368504231203130"
},
{
"DOI": "10.1097/01.shk.0000208808.03148.ea",
"doi-asserted-by": "publisher",
"key": "bibr20-00368504231203130"
},
{
"DOI": "10.1097/CCM.0b013e3182592006",
"doi-asserted-by": "publisher",
"key": "bibr21-00368504231203130"
},
{
"DOI": "10.1097/SHK.0b013e3182894016",
"doi-asserted-by": "publisher",
"key": "bibr22-00368504231203130"
},
{
"DOI": "10.1097/00024382-199803000-00002",
"doi-asserted-by": "publisher",
"key": "bibr23-00368504231203130"
},
{
"DOI": "10.1371/journal.pone.0189536",
"doi-asserted-by": "publisher",
"key": "bibr24-00368504231203130"
},
{
"DOI": "10.1378/chest.128.5.3159",
"doi-asserted-by": "publisher",
"key": "bibr25-00368504231203130"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.sagepub.com/doi/10.1177/00368504231203130"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effects of nebulized hypertonic saline on inflammatory mediators in patients with severe COVID-19 pneumonia: A double-blinded randomized controlled trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1177/sage-journals-update-policy",
"volume": "106"
}