Unveiling patenting strategies of therapeutics and vaccines: evergreening in the context of COVID-19 pandemic
et al., Frontiers in Medicine, doi:10.3389/fmed.2023.1287542, Dec 2023
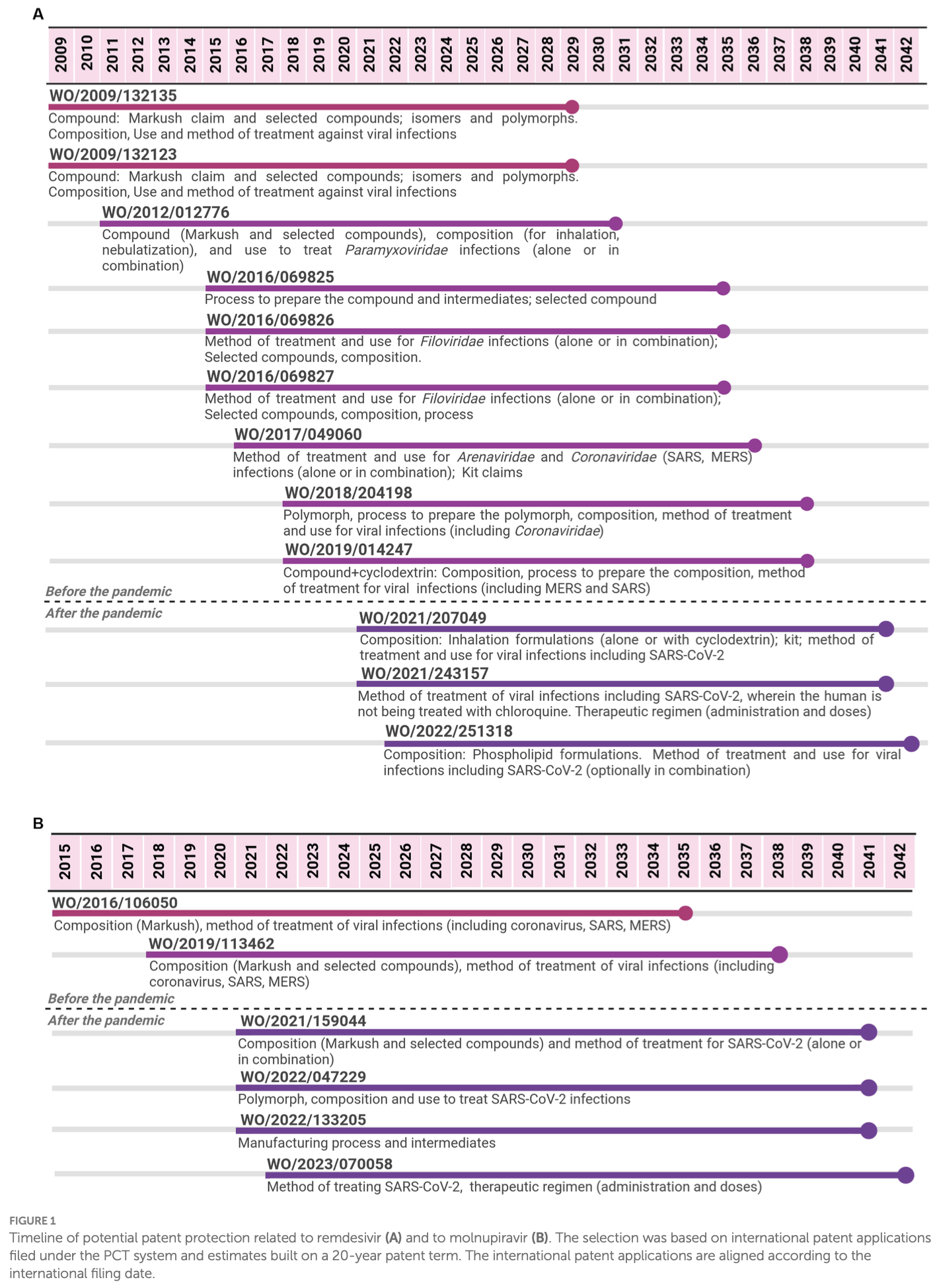
Review of the patenting activity and evergreening approaches for three major COVID-19 antiviral medications - remdesivir, molnupiravir, and favipiravir. Authors found extensive primary and secondary patent filing, with 29 applications covering up to 43 years of potential monopoly protections, with an intensification of evergreening efforts during the pandemic, showing attempts to leverage follow-on patents to extend and maintain monopolies. New patents had limited effect for favipiravir where inexpensive generics were available. Expansive patenting creates disincentives for studying or promoting cheaper off-patent treatment alternatives, biases R&D priorities towards patented options, and erects legal barriers to generic competition even after primary patents expire.
Review covers molnupiravir, remdesivir, and favipiravir.
1.
Shen et al., Carboxylesterase Factors Influencing the Therapeutic Activity of Common Antiviral Medications Used for SARS-CoV-2 Infection, Pharmaceutics, doi:10.3390/pharmaceutics17070832.
2.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
3.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
Bacigalupo et al., 6 Dec 2023, peer-reviewed, 6 authors.
Contact: mariaflorenciapignataro@gmail.com.
Unveiling patenting strategies of therapeutics and vaccines: evergreening in the context of COVID-19 pandemic
Frontiers in Medicine, doi:10.3389/fmed.2023.1287542
In the pharmaceutical sector, evergreening is considered a range of practices applied to extend monopoly protection on existing products. Filing several patent applications related to the same active pharmaceutical ingredient (API) is one of the most common manifestations of evergreening. During the COVID-19 pandemic, several health technologies were developed. This study aimed to analyze the extension of evergreening for selected health technologies for SARS-CoV-2 through patent filing strategies. Starting with the selection of three antivirals, one biological and two vaccines, a patent landscape was built based on public and private databases. Regarding these selected technologies, we analyzed some of the evergreening strategies used by different applicants, academic institutions or pharmaceutical companies and found a total of 29 applications (10 after the pandemic) for antivirals, 3 applications for a biological drug (1 after the pandemic), and 41 applications for vaccines (23 after the pandemic). Despite differences among the technologies, a common aspect found in all analyzed cases is the intense patent filing after the pandemic, aligned to the fact that those technologies were moving through the R&D process up to regulatory approval. The evergreening approach pursued has already been found in other diseases, with the risk of monopoly extension and also bringing legal uncertainty due to the lack of transparency of newer patent applications covering specific medical indications. Therefore, efforts to address evergreening should be pursued by countries, including the adoption of a public health approach to the patent examination of those technologies to prevent the granting of undeserved patents.
Author contributions
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Banerjee, Banerjee, Singh, Kumar, Pooja et al., A clinical insight on new discovered molecules and repurposed drugs for the treatment of COVID-19, Vaccines, doi:10.3390/vaccines11020332
Benenato, Kumarasinghe, Cornebise, Inventors, Modernatx, Inc, applicant. Compounds and compositions for intracellular delivery of therapeutic agents, WO
Chaves, Vieira, Rdfda, Vianna, Medicines under exclusivity situation funded by the Ministry of Health: analysis of the patent situation and public 10
Chesbrough, Recovering abandoned compounds through expanded external IP licensing, Calif Manag Rev, doi:10.1525/cmr.2013.55.4.83
Correa, Guidelines for the examination of patent applications relating to pharmaceuticals
Cross, Rho, Reddy, Pepperrell, Rodgers et al., Who funded the research behind the Oxford-AstraZeneca COVID-19 vaccine?, BMJ Glob Health, doi:10.1136/bmjgh-2021-007321
Daerden, Moderna's profits show why big pharma can't meet our health needs
Forbes, The global 2000
Garrison, How the 'Oxford' Covid-19 vaccine became the ' AstraZeneca' Covid-19 vaccine
Guy, Dp, Romanelli, Dutch, Rapid repurposing of drugs for COVID-19, Science, doi:10.1126/science.abb9332
Hatchett, Saville, Downham, Cueni, Bigger et al., Towards vaccinating the world: landscape of current COVID-19 supply chain manufacturing capacity, potential challenges, initial responses, and possible "solution space
Hill, Wang, Levi, Heath, Fortunak, Minimum costs to manufacture new treatments for COVID-19, J Virus Erad, doi:10.1016/S2055-6640(20)30018-2
Holy, Rosenberg, Inventors
Kapczynski, Park, Sampat, Polymorphs and prodrugs and salts (oh my!): an empirical analysis of "secondary" pharmaceutical patents, PLoS One, doi:10.1371/journal.pone.0049470.t001
Kondratyuk, Access to Oxford-AstraZeneca, Johnson and Johnson, Moderna and Pfizer-BioNTech COVID-19 vaccines in 17 middle-income countries in 2021
Krishnamurthy, Grimshaw, Axson, Choe, Miller, Drug repurposing: a systematic review on root causes, barriers and facilitators, BMC Health Serv Res, doi:10.1186/s12913-022-08272-z
Labban, The case of remdesivir: how do you calculate the cost of a pandemic drug? Pharmaceutical technology
Lalani, Nagar, Sarpatwari, Barenie, Avorn et al., US public investment in development of mRNA covid-19 vaccines: retrospective cohort study, BMJ, doi:10.1136/bmj-2022-073747
Lamontagne, Agarwal, Rochwerg, Siemieniuk, Agoritsas et al., A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Lo, Jordan, Arvey, Sudhamsu, Shrivastava-Ranjan et al., GS-5734 and its parent nucleoside analog inhibit filo-, Pneumo-, and paramyxoviruses, Sci Rep, doi:10.1038/srep43395
Lurie, Keusch, Dzau, Urgent lessons from COVID 19: why the world needs a standing, coordinated system and sustainable financing for global research and development, Lancet, doi:10.1016/S0140-6736(21)00503-1
Medicines, COVID-19: another of Gilead's five patent applications on remdesivir is opposed in Argentina
Medicines, Pool, Medspal, None
Medicines, Pool, None
Medicines, Pool, Vaxpal, None
Medicines, Thai civil society opposes patent on an influenza drug, now used for COVID-19
Moon, Ruiz, Vieira, Averting future vaccine injustice, N Engl J Med, doi:10.1056/NEJMp2107528
Oliyai, Fardis, 05 -Viread
Pilkington, Keestra, Hill, Global COVID-19 vaccine inequity: failures in the first year of distribution and potential solutions for the future, Front Public Health, doi:10.3389/fpubh.2022.821117
Pollack, High cost of Sovaldi hepatitis C drug prompts a call to void its patents, The New York Times
Reynolds, US gets almost all of the world's supply of key Covid-19 drug
Rubin, Chan-Tack, Farley, Sherwat, FDA approval of remdesivir: a step in the right direction, N Engl J Med, doi:10.1056/NEJMp2032369
Scopel, Estratégias de acumulação de capital das big pharma: estudo de empresas selecionadas entre 2008 e 2019
Sheahan, Sims, Graham, Menachery, Gralinski et al., Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses, Sci Transl Med, doi:10.1126/scitranslmed.aal3653
Shores, The mRNA patent and competitive landscape: pioneers, litigation outlook and big pharma's next moves (part III). IPWatchdogcom Patents & Intellectual Property Law
Singla, Goyal, Antiviral activity of molnupiravir against COVID-19: a schematic review of evidences, Bull Natl Res Cent, doi:10.1186/s42269-022-00753-9
Storz, The COVID-19 vaccine patent race, Nat Biotechnol, doi:10.1038/s41587-022-01376-1
Thambisetty, Mcmahon, Mcdonagh, Kang, Dutfield, Addressing vaccine inequity during the COVID-19 pandemic: the trips intellectual property waiver proposal and beyond, Camb Law J, doi:10.1017/S0008197322000241
Warry, Coronavirus: US buys nearly all of Gilead's Covid-19 drug remdesivir
DOI record:
{
"DOI": "10.3389/fmed.2023.1287542",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2023.1287542",
"abstract": "<jats:p>In the pharmaceutical sector, evergreening is considered a range of practices applied to extend monopoly protection on existing products. Filing several patent applications related to the same active pharmaceutical ingredient (API) is one of the most common manifestations of evergreening. During the COVID-19 pandemic, several health technologies were developed. This study aimed to analyze the extension of evergreening for selected health technologies for SARS-CoV-2 through patent filing strategies. Starting with the selection of three antivirals, one biological and two vaccines, a patent landscape was built based on public and private databases. Regarding these selected technologies, we analyzed some of the evergreening strategies used by different applicants, academic institutions or pharmaceutical companies and found a total of 29 applications (10 after the pandemic) for antivirals, 3 applications for a biological drug (1 after the pandemic), and 41 applications for vaccines (23 after the pandemic). Despite differences among the technologies, a common aspect found in all analyzed cases is the intense patent filing after the pandemic, aligned to the fact that those technologies were moving through the R&amp;D process up to regulatory approval. The evergreening approach pursued has already been found in other diseases, with the risk of monopoly extension and also bringing legal uncertainty due to the lack of transparency of newer patent applications covering specific medical indications. Therefore, efforts to address evergreening should be pursued by countries, including the adoption of a public health approach to the patent examination of those technologies to prevent the granting of undeserved patents.</jats:p>",
"alternative-id": [
"10.3389/fmed.2023.1287542"
],
"author": [
{
"affiliation": [],
"family": "Bacigalupo",
"given": "María Lorena",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pignataro",
"given": "María Florencia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scopel",
"given": "Carolinne Thays",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kondratyuk",
"given": "Sergiy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mellouk",
"given": "Othoman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaves",
"given": "Gabriela Costa",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
12,
6
]
],
"date-time": "2023-12-06T08:36:06Z",
"timestamp": 1701851766000
},
"deposited": {
"date-parts": [
[
2023,
12,
6
]
],
"date-time": "2023-12-06T08:36:10Z",
"timestamp": 1701851770000
},
"indexed": {
"date-parts": [
[
2023,
12,
7
]
],
"date-time": "2023-12-07T00:52:09Z",
"timestamp": 1701910329940
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12,
6
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
6
]
],
"date-time": "2023-12-06T00:00:00Z",
"timestamp": 1701820800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1287542/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
12,
6
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
6
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"key": "ref1",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(21)00503-1",
"article-title": "Urgent lessons from COVID 19: why the world needs a standing, coordinated system and sustainable financing for global research and development",
"author": "Lurie",
"doi-asserted-by": "publisher",
"first-page": "1229",
"journal-title": "Lancet",
"key": "ref2",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMp2107528",
"article-title": "Averting future vaccine injustice",
"author": "Moon",
"doi-asserted-by": "publisher",
"first-page": "193",
"journal-title": "N Engl J Med",
"key": "ref3",
"volume": "385",
"year": "2021"
},
{
"author": "Hatchett",
"key": "ref4",
"volume-title": "Towards vaccinating the world: landscape of current COVID-19 supply chain manufacturing capacity, potential challenges, initial responses, and possible “solution space”: a discussion document (appendix)",
"year": "2021"
},
{
"DOI": "10.1136/bmj-2022-073747",
"article-title": "US public investment in development of mRNA covid-19 vaccines: retrospective cohort study",
"author": "Lalani",
"doi-asserted-by": "publisher",
"first-page": "e073747",
"journal-title": "BMJ",
"key": "ref5",
"volume": "380",
"year": "2023"
},
{
"DOI": "10.1136/bmjgh-2021-007321",
"article-title": "Who funded the research behind the Oxford-AstraZeneca COVID-19 vaccine?",
"author": "Cross",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Glob Health",
"key": "ref6",
"volume": "6",
"year": "2021"
},
{
"article-title": "COVID-19 vaccine R&D investments",
"key": "ref7",
"year": "2021"
},
{
"DOI": "10.1017/S0008197322000241",
"article-title": "Addressing vaccine inequity during the COVID-19 pandemic: the trips intellectual property waiver proposal and beyond",
"author": "Thambisetty",
"doi-asserted-by": "publisher",
"first-page": "384",
"journal-title": "Camb Law J",
"key": "ref8",
"volume": "81",
"year": "2022"
},
{
"article-title": "Global vaccine market report 2022: a shared understanding for equitable access to vaccines",
"key": "ref9",
"year": "2023"
},
{
"author": "Daerden",
"key": "ref10",
"year": "2022"
},
{
"key": "ref11",
"year": "2023"
},
{
"author": "Scopel",
"key": "ref12",
"volume-title": "Estratégias de acumulação de capital das big pharma: estudo de empresas selecionadas entre 2008 e 2019",
"year": "2023"
},
{
"author": "Kondratyuk",
"key": "ref13",
"volume-title": "Access to Oxford-AstraZeneca, Johnson and Johnson, Moderna and Pfizer-BioNTech COVID-19 vaccines in 17 middle-income countries in 2021",
"year": "2023"
},
{
"key": "ref14",
"year": "2023"
},
{
"key": "ref15",
"year": ""
},
{
"key": "ref16",
"year": "2021"
},
{
"key": "ref17",
"year": "2021"
},
{
"key": "ref18",
"year": "2020"
},
{
"key": "ref19",
"year": "2021"
},
{
"key": "ref20",
"year": "2020"
},
{
"DOI": "10.1038/s41587-022-01376-1",
"article-title": "The COVID-19 vaccine patent race",
"author": "Storz",
"doi-asserted-by": "publisher",
"first-page": "1001",
"journal-title": "Nat Biotechnol",
"key": "ref21",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1016/S2055-6640(20)30018-2",
"article-title": "Minimum costs to manufacture new treatments for COVID-19",
"author": "Hill",
"doi-asserted-by": "publisher",
"first-page": "61",
"journal-title": "J Virus Erad",
"key": "ref22",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0049470.t001",
"article-title": "Polymorphs and prodrugs and salts (oh my!): an empirical analysis of “secondary” pharmaceutical patents",
"author": "Kapczynski",
"doi-asserted-by": "publisher",
"first-page": "e49470",
"journal-title": "PLoS One",
"key": "ref23",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1136/bmj.m3379",
"article-title": "A living WHO guideline on drugs for covid-19",
"author": "Lamontagne",
"doi-asserted-by": "publisher",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "ref24",
"volume": "370",
"year": "2020"
},
{
"key": "ref25",
"year": "2023"
},
{
"key": "ref26",
"year": "2023"
},
{
"key": "ref27",
"year": "2023"
},
{
"author": "Correa",
"key": "ref28",
"year": "2016"
},
{
"author": "Garrison",
"key": "ref29",
"year": "2020"
},
{
"author": "Benenato",
"key": "ref30",
"year": "2017"
},
{
"DOI": "10.1038/srep43395",
"article-title": "GS-5734 and its parent nucleoside analog inhibit filo-, Pneumo-, and paramyxoviruses",
"author": "Lo",
"doi-asserted-by": "publisher",
"first-page": "43395",
"journal-title": "Sci Rep",
"key": "ref31",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1126/scitranslmed.aal3653",
"article-title": "Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses",
"author": "Sheahan",
"doi-asserted-by": "publisher",
"first-page": "eaal3653",
"journal-title": "Sci Transl Med",
"key": "ref32",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1126/science.abb9332",
"article-title": "Rapid repurposing of drugs for COVID-19",
"author": "Guy",
"doi-asserted-by": "publisher",
"first-page": "829",
"journal-title": "Science",
"key": "ref33",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1186/s42269-022-00753-9",
"article-title": "Antiviral activity of molnupiravir against COVID-19: a schematic review of evidences",
"author": "Singla",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Bull Natl Res Cent",
"key": "ref34",
"volume": "46",
"year": "2022"
},
{
"DOI": "10.3390/vaccines11020332",
"article-title": "A clinical insight on new discovered molecules and repurposed drugs for the treatment of COVID-19",
"author": "Banerjee",
"doi-asserted-by": "publisher",
"first-page": "332",
"journal-title": "Vaccines",
"key": "ref35",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1186/s12913-022-08272-z",
"article-title": "Drug repurposing: a systematic review on root causes, barriers and facilitators",
"author": "Krishnamurthy",
"doi-asserted-by": "publisher",
"first-page": "970",
"journal-title": "BMC Health Serv Res",
"key": "ref36",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1525/cmr.2013.55.4.83",
"article-title": "Recovering abandoned compounds through expanded external IP licensing",
"author": "Chesbrough",
"doi-asserted-by": "publisher",
"first-page": "83",
"journal-title": "Calif Manag Rev",
"key": "ref37",
"volume": "55",
"year": "2013"
},
{
"key": "ref38",
"year": "2022"
},
{
"key": "ref39",
"year": "2002"
},
{
"author": "Pollack",
"key": "ref40",
"year": "2015"
},
{
"article-title": "Medicines under exclusivity situation funded by the Ministry of Health: analysis of the patent situation and public procurement",
"author": "Chaves",
"key": "ref41",
"year": "2019"
},
{
"author": "Reynolds",
"key": "ref42",
"year": "2020"
},
{
"author": "Warry",
"key": "ref43",
"year": "2020"
},
{
"author": "Labban",
"key": "ref44",
"year": "2020"
},
{
"DOI": "10.1016/B0-08-045044-X/00289-3",
"article-title": "8.05 - Viread",
"author": "Oliyai",
"doi-asserted-by": "crossref",
"first-page": "53",
"key": "ref45",
"volume-title": "Comprehensive medicinal chemistry II",
"year": "2007"
},
{
"author": "Holy",
"key": "ref46",
"year": "1989"
},
{
"DOI": "10.1056/NEJMp2032369",
"article-title": "FDA approval of remdesivir: a step in the right direction",
"author": "Rubin",
"doi-asserted-by": "publisher",
"first-page": "2598",
"journal-title": "N Engl J Med",
"key": "ref47",
"volume": "383",
"year": "2020"
},
{
"key": "ref48",
"year": "1994"
},
{
"key": "ref49",
"year": "2021"
},
{
"key": "ref50",
"year": "2017"
},
{
"article-title": "The mRNA patent and competitive landscape: pioneers, litigation outlook and big pharma’s next moves (part III)",
"author": "Shores",
"key": "ref51",
"volume-title": "IPWatchdogcom",
"year": "2021"
},
{
"DOI": "10.3389/fpubh.2022.821117",
"article-title": "Global COVID-19 vaccine inequity: failures in the first year of distribution and potential solutions for the future",
"author": "Pilkington",
"doi-asserted-by": "publisher",
"first-page": "821117",
"journal-title": "Front Public Health",
"key": "ref52",
"volume": "10",
"year": "2022"
}
],
"reference-count": 52,
"references-count": 52,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1287542/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Unveiling patenting strategies of therapeutics and vaccines: evergreening in the context of COVID-19 pandemic",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "10"
}
bacigalupo