Feb 21 |
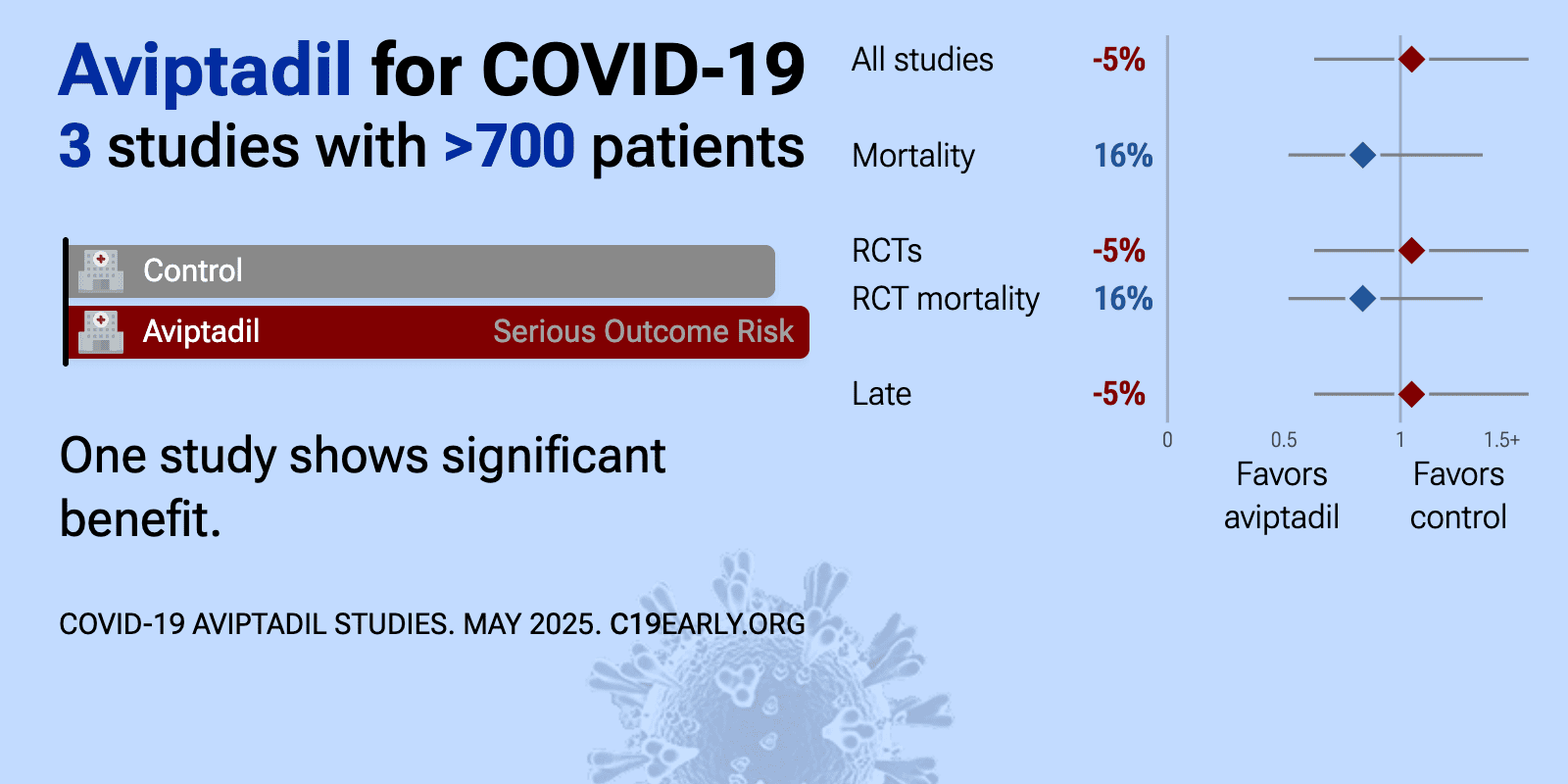
Meta-analysis of aviptadil studies | |
| Meta-analysis of aviptadil studies | ||
Sep 30 2023 |
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(23)00147-9 | Intravenous aviptadil and remdesivir for treatment of COVID-19-associated hypoxaemic respiratory failure in the USA (TESICO): a randomised, placebo-controlled trial |
| 6% higher mortality (p=0.71) and 10% lower progression (p=0.54). RCT 461 hospitalized patients with COVID-19-associated respiratory failure showing no significant difference in outcomes with aviptadil treatment. | ||
Aug 29 2022 |
et al., Critical Care Medicine, doi:10.1097/CCM.0000000000005660 | The Use of IV Vasoactive Intestinal Peptide (Aviptadil) in Patients With Critical COVID-19 Respiratory Failure: Results of a 60-Day Randomized Controlled Trial |
| 35% lower mortality (p=0.04) and 20% lower progression (p=0.27). RCT 196 patients with critical COVID-19 respiratory failure showing improved survival but no significant difference in the primary endpoint of "alive and free of respiratory failure at day 60" with IV aviptadil. | ||
Mar 31 2022 |
et al., NCT04488081 | I-SPY COVID Trial Sponsored by Quantum Leap Healthcare Collaborative Suggests No Clinical Benefit with Addition of Nebulized ZYESAMI® (aviptadil) When Given by Mouth Inhalation in Critically Ill Patients with COVID-19 |
| 79% worse recovery (p=0.02). RCT 118 patients showing significantly worse recovery with aviptadil treatment. | ||