Efficacy and safety of oral melatonin in patients with severe COVID-19: a randomized controlled trial
et al., Inflammopharmacology, doi:10.1007/s10787-022-01096-7, Nov 2022
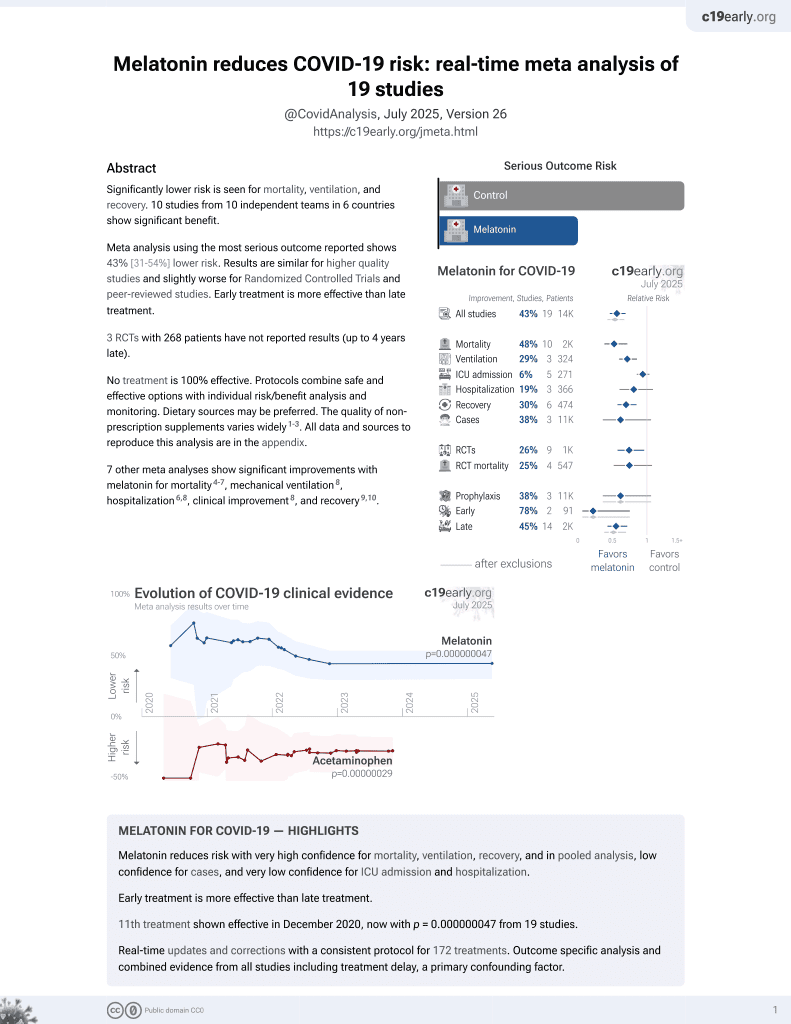
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 226 ICU patients in Iran, showing lower mortality with melatonin treatment.
|
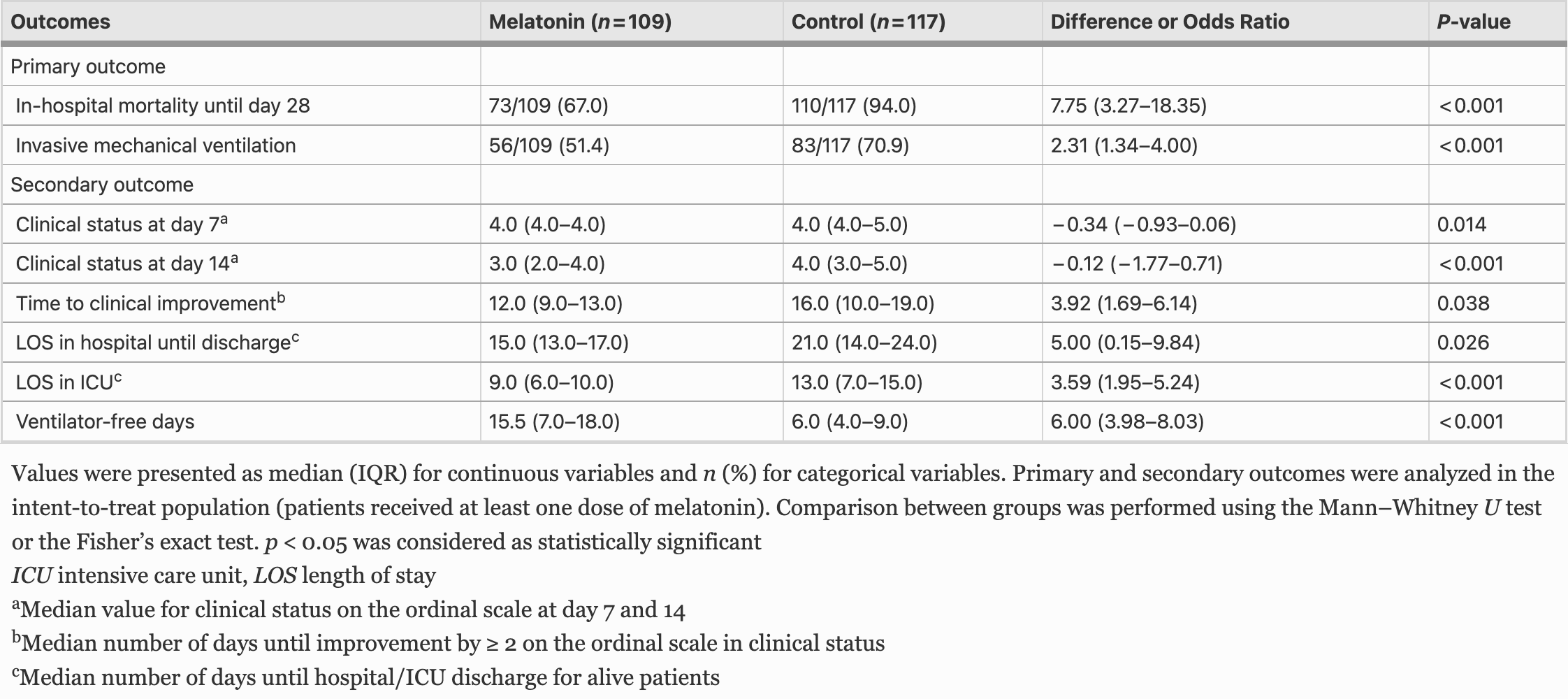
risk of death, 28.8% lower, RR 0.71, p < 0.001, treatment 73 of 109 (67.0%), control 110 of 117 (94.0%), NNT 3.7, primary outcome.
|
|
risk of mechanical ventilation, 27.6% lower, RR 0.72, p = 0.003, treatment 56 of 109 (51.4%), control 83 of 117 (70.9%), NNT 5.1, primary outcome.
|
|
clinical status, 25.0% lower, RR 0.75, p = 0.001, treatment 109, control 117, day 14.
|
|
recovery time, 25.0% lower, relative time 0.75, p = 0.04, treatment 109, control 117.
|
|
hospitalization time, 28.6% lower, relative time 0.71, p = 0.03, treatment 109, control 117.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ameri et al., 19 Nov 2022, Randomized Controlled Trial, Iran, peer-reviewed, 9 authors, study period 1 March, 2021 - 30 November, 2021.
Efficacy and safety of oral melatonin in patients with severe COVID-19: a randomized controlled trial
Inflammopharmacology, doi:10.1007/s10787-022-01096-7
Patients with COVID-19 have shown melatonin deficiency. We evaluated the efficacy and safety of administration oral melatonin in patients with COVID-19-induced pneumonia. Patients were randomly assigned in a 1:1 ratio to receive melatonin plus standard treatment or standard treatment alone. The primary outcomes were mortality rate and requirement of IMV. The clinical status of patients was recorded at baseline and every day over hospitalization based on seven-category ordinal scale from 1 (discharged) to 7 (death). A total of 226 patients (109 in the melatonin group and 117 in the control group) were enrolled (median age; in melatonin group: 54.60 ± 11.51, in control group: 54.69 ± 13.40). The mortality rate was 67% in the melatonin group and 94% in the control group (OR; 7.75, 95% CI, 3.27-18.35, P < 0.001). The rate of IMV requirement was 51.4% in the melatonin group and 70.9% in the control group, for an OR of 2.31 (95% CI, 1.34-4.00, P < 0.001). The median number of days to hospital discharge was 15 days (13-17) in the melatonin group and 21 days (14-24) in the control group (OR; 5.00, 95% CI, 0.15-9.84, P = 0.026). Time to clinical status improvement by ≥ 2 on the ordinal scale in was 12 days (9-13) in the melatonin group and 16 days (10-19) in the control group (OR; 3.92, 95% CI, 1.69-6.14, P = 0.038). Melatonin significantly improved clinical status with a safe profile in patients with severe COVID-19 pneumonia.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s10787-022-01096-7. Author contributions AA, AZ, and MF: conceptualization, methodology, software. MF, MM, and SH: data curation, writing-original draft preparation. MF, MV, OS, and MK: visualization, investigation. AAi: supervision. SH: software, validation. MF: writing-reviewing and editing.
Declarations Conflict of interest None of the authors have a conflict of interest to disclose. Ethical approval and consent to participate Ethics approval was obtained from the ethics committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1399.526). The study was also undertaken in accordance with the guidelines of the Declaration of Helsinki and the principles of the International Conference on Harmonization Good Clinical Practice.
Consent for publication A written informed consent was obtained from all patients. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
Aisa-Alvarez, Soto, Guarner-Lans, Camarena-Alejo, Franco-Granillo et al., Usefulness of antioxidants as adjuvant therapy for septic shock a randomized clinical trial, Medicina (kaunas)
Al-Samkari, Leaf, Dzik, Carlson, Fogerty et al., COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection, Blood
Alamili, Bendtzen, Lykkesfeldt, Rosenberg, Gögenur, Melatonin suppresses markers of inflammation and oxidative damage in a human daytime endotoxemia model, J Crit Care
Anderson, Maes, Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications, Curr Top Med Chem
Anderson, Reiter, Melatonin: roles in influenza, Covid-19, and other viral infections, Rev Med Virol
Bahrampourjuybari, Pourhanifeh, Hosseinzadeh, Hemati, Mehrzadi, Melatonin potentials against viral infections including COVID-19: current evidence and new findings, Virus Res
Baloch, Baloch, Zheng, Pei, The Coronavirus disease 2019 (COVID-19) Pandemic, Tohoku J Exp Med
Bonilla, Valero, Chacín-Bonilla, Medina-Leendertz, Melatonin and viral infections, J Pineal Res
Bouck, Denorme, Holle, Middelton, Blair et al., COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity, Arterioscler Thromb Vasc Biol
Camp, Bai, Gonullu, Nayak, Hm, Melatonin interferes with COVID-19 at several distinct ROS-related steps, J Inorg Biochem
Can, Ulugöl, Güneş, Aksu, Tosun et al., Effects of alprazolam and melatonin used for premedication on oxidative stress, glicocalyx integrity and neurocognitive functions, Turk J Anaesthesiol Reanim
Cichoz-Lach, Celinski, Konturek, Konturek, Slomka, The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis, J Physiol Pharmacol
Cook, Mascolo, Bass, Duffy, Zehring et al., Has COVID-19 Complicated eating disorder treatment? An examination of comorbidities and treatment response before and during the COVID-19 pandemic, Prim Care Companion CNS Disord
Davoudi-Monfared, Rahmani, Khalili, Hajiabdolbaghi, Salehi et al., A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19, Antimicrob Agents Chemother
Dequin, Heming, Meziani, Plantefève, Voiriot et al., Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients With COVID-19: a randomized clinical trial, JAMA
Dianatkhah, Najafi, Sharifzadeh, Ahmadi, Sharifnia et al., Melatonin supplementation may improve the outcome of patients with hemorrhagic stroke in the intensive care unit, J Res Pharm Pract
Fogleman, Cohen, Mercier, Farrell, Rutz et al., A pilot of a randomized control trial of melatonin and vitamin C for mild-to-moderate COVID-19, J Am Board Fam Med
Foley, Steel, Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence, Complement Ther Med
Galley, Lowes, Allen, Cameron, Aucott et al., Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis, J Pineal Res
Gitto, Karbownik, Reiter, Tan, Cuzzocrea et al., Effects of melatonin treatment in septic newborns, Pediatr Res
Hasan, Atrakji, Mehuaiden, The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients, Int J Infect Dis
Henderson, Kim, Lee, Use of melatonin as adjunctive therapy in neonatal sepsis: a systematic review and meta-analysis, Complement Ther Med
Iversen, Dahm, Skretting, Mowinckel, Stranda et al., Reduced peak, but no diurnal variation, in thrombin generation upon melatonin supplementation in tetraplegia. A randomised, placebo-controlled study, Thromb Haemost
Kleszczyński, Slominski, Steinbrink, Reiter, Clinical trials for use of melatonin to fight against COVID-19 are urgently needed, Nutrients
Kronstein-Wiedemann, Stadtmüller, Traikov, Georgi, Teichert et al., SARS-CoV-2 infects red blood cell progenitors and dysregulates hemoglobin and iron metabolism, Stem Cell Rev Rep
Lissoni, Modulation of anticancer cytokines IL-2 and IL-12 by melatonin and the other pineal indoles 5-methoxytryptamine and 5-methoxytryptophol in the treatment of human neoplasms, Ann N Y Acad Sci
Marzougui, Hammouda, Bendhia, Maaloul, Agrebi et al., Melatonin ingestion before intradialytic exercise improves immune responses in hemodialysis patients, Int Urol Nephrol
Mazeraud, Jamme, Mancusi, Latroche, Megarbane et al., Intravenous immunoglobulins in patients with COVID-19-associated moderate-tosevere acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial, Lancet Respir Med
Nair, Perioperative melatonin in COVID-19 patients: benefits beyond sedation and analgesia, Med Gas Res
Rahmani, Davoudi-Monfared, Nourian, Khalili, Hajizadeh et al., Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial, Int Immunopharmacol
Reiter, Ma, Sharma, Treatment of ebola and other infectious diseases: melatonin "goes viral, Melatonin Res
Rodríguez-Rubio, Figueira, Acuña-Castroviejo, Borobia, Escames et al., A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial, Arch Med Res
Seely, Legacy, Auer, Fazekas, Delic et al., Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): a randomized placebo controlled clinical trial, EClinicalMedicine
Sen, Deficient synthesis of melatonin in COVID-19 can impair the resistance of coronavirus patients to mucormycosis, Med Hypotheses
Shiu, Reiter, Tan, Pang, Urgent search for safe and effective treatments of severe acute respiratory syndrome: is melatonin a promising candidate drug?, J Pineal Res
Shneider, Kudriavtsev, Vakhrusheva, Can melatonin reduce the severity of COVID-19 pandemic?, Int Rev Immunol
Wirtz, Bärtschi, Spillmann, Ehlert, Känel, Effect of oral melatonin on the procoagulant response to acute psychosocial stress healthy men: a randomized placebo-controlled study, J Pineal Res
Zhang, Li, Grailer, Wang, Wang et al., Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome, J Pineal Res
Zhang, Wang, Ni, Di, Ma et al., COVID-19: melatonin as a potential adjuvant treatment, Life Sci
Zisapel, New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation, Br J Pharmacol
DOI record:
{
"DOI": "10.1007/s10787-022-01096-7",
"ISSN": [
"0925-4692",
"1568-5608"
],
"URL": "http://dx.doi.org/10.1007/s10787-022-01096-7",
"alternative-id": [
"1096"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "6 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "19 November 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "None of the authors have a conflict of interest to disclose."
},
{
"group": {
"label": "Ethical approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Ethics approval was obtained from the ethics committee of Hormozgan University of Medical Sciences (<u>IR.HUMS.REC.1399.526</u>). The study was also undertaken in accordance with the guidelines of the Declaration of Helsinki and the principles of the International Conference on Harmonization Good Clinical Practice."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "A written informed consent was obtained from all patients."
}
],
"author": [
{
"affiliation": [],
"family": "Ameri",
"given": "Ali",
"sequence": "first"
},
{
"affiliation": [],
"family": "Frouz Asadi",
"given": "Masoomeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ziaei",
"given": "Ava",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vatankhah",
"given": "Majid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Safa",
"given": "Omid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamali",
"given": "Manoochehr",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4568-7024",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fathalipour",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahmoodi",
"given": "Masoumeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassanipour",
"given": "Soheil",
"sequence": "additional"
}
],
"container-title": "Inflammopharmacology",
"container-title-short": "Inflammopharmacol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
19
]
],
"date-time": "2022-11-19T07:03:07Z",
"timestamp": 1668841387000
},
"deposited": {
"date-parts": [
[
2022,
11,
19
]
],
"date-time": "2022-11-19T21:38:42Z",
"timestamp": 1668893922000
},
"funder": [
{
"DOI": "10.13039/501100011917",
"award": [
"990592"
],
"doi-asserted-by": "publisher",
"name": "Hormozgan University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2022,
11,
20
]
],
"date-time": "2022-11-20T05:41:10Z",
"timestamp": 1668922870925
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
19
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
19
]
],
"date-time": "2022-11-19T00:00:00Z",
"timestamp": 1668816000000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
19
]
],
"date-time": "2022-11-19T00:00:00Z",
"timestamp": 1668816000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-022-01096-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s10787-022-01096-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-022-01096-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
11,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
19
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3390/medicina56110619",
"author": "A Aisa-Alvarez",
"doi-asserted-by": "publisher",
"first-page": "619",
"journal-title": "Medicina (kaunas)",
"key": "1096_CR1",
"unstructured": "Aisa-Alvarez A, Soto ME, Guarner-Lans V, Camarena-Alejo G, Franco-Granillo J, Martínez-Rodríguez EA, Gamboa Ávila R, Manzano Pech L, Pérez-Torres I (2020) Usefulness of antioxidants as adjuvant therapy for septic shock a randomized clinical trial. Medicina (kaunas) 56:619",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020006520",
"author": "H Al-Samkari",
"doi-asserted-by": "publisher",
"first-page": "489",
"journal-title": "Blood",
"key": "1096_CR2",
"unstructured": "Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP (2020) COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 136:489–500",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2013.09.006",
"author": "M Alamili",
"doi-asserted-by": "publisher",
"first-page": "184.e9",
"journal-title": "J Crit Care",
"key": "1096_CR3",
"unstructured": "Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gögenur I (2014) Melatonin suppresses markers of inflammation and oxidative damage in a human daytime endotoxemia model. J Crit Care 29:184.e9-184.e13",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.2174/1568026620666200131094445",
"author": "G Anderson",
"doi-asserted-by": "publisher",
"first-page": "524",
"journal-title": "Curr Top Med Chem",
"key": "1096_CR4",
"unstructured": "Anderson G, Maes M (2020) Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr Top Med Chem 20:524–539",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2109",
"author": "G Anderson",
"doi-asserted-by": "publisher",
"journal-title": "Rev Med Virol",
"key": "1096_CR5",
"unstructured": "Anderson G, Reiter RJ (2020) Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol 30:e2109",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.virusres.2020.198108",
"author": "K BahrampourJuybari",
"doi-asserted-by": "publisher",
"first-page": "198108",
"journal-title": "Virus Res",
"key": "1096_CR6",
"unstructured": "BahrampourJuybari K, Pourhanifeh MH, Hosseinzadeh A, Hemati K, Mehrzadi S (2020) Melatonin potentials against viral infections including COVID-19: current evidence and new findings. Virus Res 287:198108",
"volume": "287",
"year": "2020"
},
{
"DOI": "10.1620/tjem.250.271",
"author": "S Baloch",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Tohoku J Exp Med",
"key": "1096_CR7",
"unstructured": "Baloch S, Baloch MA, Zheng T, Pei X (2020) The Coronavirus disease 2019 (COVID-19) Pandemic. Tohoku J Exp Med 250:271–278",
"volume": "250",
"year": "2020"
},
{
"DOI": "10.1046/j.1600-079X.2003.00105.x",
"author": "E Bonilla",
"doi-asserted-by": "publisher",
"first-page": "73",
"journal-title": "J Pineal Res",
"key": "1096_CR8",
"unstructured": "Bonilla E, Valero N, Chacín-Bonilla L, Medina-Leendertz S (2004) Melatonin and viral infections. J Pineal Res 36:73–79",
"volume": "36",
"year": "2004"
},
{
"author": "EG Bouck",
"first-page": "401",
"journal-title": "Arterioscler Thromb Vasc Biol",
"key": "1096_CR9",
"unstructured": "Bouck EG, Denorme F, Holle LA, Middelton EA, Blair AM, de Laat B, Schiffman JD, Yost CC, Rondina MT, Wolberg AS, Campbell RA (2021) COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol 41:401–414",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1016/j.jinorgbio.2021.111546",
"author": "OG Camp",
"doi-asserted-by": "publisher",
"journal-title": "J Inorg Biochem",
"key": "1096_CR10",
"unstructured": "Camp OG, Bai D, Gonullu DC, Nayak N, Abu-Soud HM (2021) Melatonin interferes with COVID-19 at several distinct ROS-related steps. J Inorg Biochem 223:111546",
"volume": "223",
"year": "2021"
},
{
"author": "MG Can",
"first-page": "233",
"journal-title": "Turk J Anaesthesiol Reanim",
"key": "1096_CR11",
"unstructured": "Can MG, Ulugöl H, Güneş I, Aksu U, Tosun M, Karduz G, Vardar K, Toraman F (2018) Effects of alprazolam and melatonin used for premedication on oxidative stress, glicocalyx integrity and neurocognitive functions. Turk J Anaesthesiol Reanim 46:233–237",
"volume": "46",
"year": "2018"
},
{
"author": "H Cichoz-Lach",
"first-page": "577",
"journal-title": "J Physiol Pharmacol",
"key": "1096_CR12",
"unstructured": "Cichoz-Lach H, Celinski K, Konturek PC, Konturek SJ, Slomka M (2010) The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J Physiol Pharmacol 61:577–580",
"volume": "61",
"year": "2010"
},
{
"DOI": "10.4088/PCC.21m03087",
"author": "B Cook",
"doi-asserted-by": "publisher",
"first-page": "21m03087",
"journal-title": "Prim Care Companion CNS Disord",
"key": "1096_CR13",
"unstructured": "Cook B, Mascolo M, Bass G, Duffy ME, Zehring B, Beasley T (2022) Has COVID-19 Complicated eating disorder treatment? An examination of comorbidities and treatment response before and during the COVID-19 pandemic. Prim Care Companion CNS Disord 24:21m03087",
"volume": "24",
"year": "2022"
},
{
"DOI": "10.1128/AAC.01061-20",
"author": "E Davoudi-Monfared",
"doi-asserted-by": "publisher",
"first-page": "e01061",
"journal-title": "Antimicrob Agents Chemother",
"key": "1096_CR14",
"unstructured": "Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, Kazemzadeh H, Yekaninejad MS (2020) A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother 64:e01061-e1120",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16761",
"author": "PF Dequin",
"doi-asserted-by": "publisher",
"first-page": "1298",
"journal-title": "JAMA",
"key": "1096_CR15",
"unstructured": "Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, François B, Aubron C, Ricard JD, Ehrmann S, Jouan Y, Guillon A, Leclerc M, Coffre C, Bourgoin H, Lengellé C, Caille-Fénérol C, Tavernier E, Zohar S, Giraudeau B, Annane D, le Gouge A (2020) Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients With COVID-19: a randomized clinical trial. JAMA 324:1298–1306",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.4103/jrpp.JRPP_17_49",
"author": "M Dianatkhah",
"doi-asserted-by": "publisher",
"first-page": "173",
"journal-title": "J Res Pharm Pract",
"key": "1096_CR16",
"unstructured": "Dianatkhah M, Najafi A, Sharifzadeh M, Ahmadi A, Sharifnia H, Mojtahedzadeh M, Najmeddin F, Moghaddas A (2017) Melatonin supplementation may improve the outcome of patients with hemorrhagic stroke in the intensive care unit. J Res Pharm Pract 6:173–177",
"volume": "6",
"year": "2017"
},
{
"DOI": "10.3122/jabfm.2022.04.210529",
"author": "C Fogleman",
"doi-asserted-by": "publisher",
"first-page": "695",
"journal-title": "J Am Board Fam Med",
"key": "1096_CR17",
"unstructured": "Fogleman C, Cohen D, Mercier A, Farrell D, Rutz J, Bresz K, Vernon T (2022) A pilot of a randomized control trial of melatonin and vitamin C for mild-to-moderate COVID-19. J Am Board Fam Med 35:695–707",
"volume": "35",
"year": "2022"
},
{
"DOI": "10.1016/j.ctim.2018.11.003",
"author": "HM Foley",
"doi-asserted-by": "publisher",
"first-page": "65",
"journal-title": "Complement Ther Med",
"key": "1096_CR18",
"unstructured": "Foley HM, Steel AE (2019) Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence. Complement Ther Med 42:65–81",
"volume": "42",
"year": "2019"
},
{
"DOI": "10.1111/jpi.12134",
"author": "HF Galley",
"doi-asserted-by": "publisher",
"first-page": "427",
"journal-title": "J Pineal Res",
"key": "1096_CR19",
"unstructured": "Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR (2014) Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res 56:427–438",
"volume": "56",
"year": "2014"
},
{
"DOI": "10.1203/00006450-200112000-00021",
"author": "E Gitto",
"doi-asserted-by": "publisher",
"first-page": "756",
"journal-title": "Pediatr Res",
"key": "1096_CR20",
"unstructured": "Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, Cordaro S, Corona G, Trimarchi G, Barberi I (2001) Effects of melatonin treatment in septic newborns. Pediatr Res 50:756–760",
"volume": "50",
"year": "2001"
},
{
"DOI": "10.1016/j.ijid.2021.10.012",
"author": "ZT Hasan",
"doi-asserted-by": "publisher",
"first-page": "79",
"journal-title": "Int J Infect Dis",
"key": "1096_CR21",
"unstructured": "Hasan ZT, Atrakji D, Mehuaiden DAK (2022) The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients. Int J Infect Dis 114:79–84",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1016/j.ctim.2018.06.002",
"author": "R Henderson",
"doi-asserted-by": "publisher",
"first-page": "131",
"journal-title": "Complement Ther Med",
"key": "1096_CR22",
"unstructured": "Henderson R, Kim S, Lee E (2018) Use of melatonin as adjunctive therapy in neonatal sepsis: a systematic review and meta-analysis. Complement Ther Med 39:131–136",
"volume": "39",
"year": "2018"
},
{
"DOI": "10.1160/TH15-05-0396",
"author": "PO Iversen",
"doi-asserted-by": "publisher",
"first-page": "964",
"journal-title": "Thromb Haemost",
"key": "1096_CR23",
"unstructured": "Iversen PO, Dahm A, Skretting G, Mowinckel MC, Stranda A, Østerud B, Sandset PM, Kostovski E (2015) Reduced peak, but no diurnal variation, in thrombin generation upon melatonin supplementation in tetraplegia. A randomised, placebo-controlled study. Thromb Haemost 114:964–968",
"volume": "114",
"year": "2015"
},
{
"DOI": "10.3390/nu12092561",
"author": "K Kleszczyński",
"doi-asserted-by": "publisher",
"first-page": "2561",
"journal-title": "Nutrients",
"key": "1096_CR24",
"unstructured": "Kleszczyński K, Slominski AT, Steinbrink K, Reiter RJ (2020) Clinical trials for use of melatonin to fight against COVID-19 are urgently needed. Nutrients 12:2561",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1007/s12015-021-10322-8",
"author": "R Kronstein-Wiedemann",
"doi-asserted-by": "publisher",
"first-page": "1809",
"journal-title": "Stem Cell Rev Rep",
"key": "1096_CR25",
"unstructured": "Kronstein-Wiedemann R, Stadtmüller M, Traikov S, Georgi M, Teichert M, Yosef H, Wallenborn J, Karl A, Schütze K, Wagner M, El-Armouche A, Tonn T (2022) SARS-CoV-2 infects red blood cell progenitors and dysregulates hemoglobin and iron metabolism. Stem Cell Rev Rep 18:1809–1821",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1111/j.1749-6632.2000.tb05421.x",
"author": "P Lissoni",
"doi-asserted-by": "publisher",
"first-page": "560",
"journal-title": "Ann N Y Acad Sci",
"key": "1096_CR26",
"unstructured": "Lissoni P (2000) Modulation of anticancer cytokines IL-2 and IL-12 by melatonin and the other pineal indoles 5-methoxytryptamine and 5-methoxytryptophol in the treatment of human neoplasms. Ann N Y Acad Sci 917:560–567",
"volume": "917",
"year": "2000"
},
{
"DOI": "10.1007/s11255-020-02643-3",
"author": "H Marzougui",
"doi-asserted-by": "publisher",
"first-page": "553",
"journal-title": "Int Urol Nephrol",
"key": "1096_CR27",
"unstructured": "Marzougui H, Hammouda O, Bendhia I, Maaloul R, Agrebi I, Chaker H, Kammoun K, Benhmida M, Ayadi F, Kallel C, Driss T, Turki M, MasmoudI H, Hachicha H (2021) Melatonin ingestion before intradialytic exercise improves immune responses in hemodialysis patients. Int Urol Nephrol 53:553–562",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00440-9",
"author": "A Mazeraud",
"doi-asserted-by": "publisher",
"first-page": "158",
"journal-title": "Lancet Respir Med",
"key": "1096_CR28",
"unstructured": "Mazeraud A, Jamme M, Mancusi RL, Latroche C, Megarbane B, Siami S, Zarka J, Moneger G, Santoli F, Argaud L, Chillet P, Muller G, Bruel C, Asfar P, Beloncle F, Reignier J, Vinsonneau C, Schimpf C, Amour J, Goulenok C, Lemaitre C, Rohaut B, Mateu P, de Rudnicki S, Mourvillier B, Declercq PL, Schwebel C, Stoclin A, Garnier M, Madeux B, Gaudry S, Bailly K, Lamer C, Aegerter P, Rieu C, Sylla K, Lucas B, Sharshar T (2022) Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 10:158–166",
"volume": "10",
"year": "2022"
},
{
"author": "AS Nair",
"first-page": "41",
"journal-title": "Med Gas Res",
"key": "1096_CR29",
"unstructured": "Nair AS (2022) Perioperative melatonin in COVID-19 patients: benefits beyond sedation and analgesia. Med Gas Res 12:41–43",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.intimp.2020.106903",
"author": "H Rahmani",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "1096_CR30",
"unstructured": "Rahmani H, Davoudi-Monfared E, Nourian A, Khalili H, Hajizadeh N, Jalalabadi NZ, Fazeli MR, Ghazaeian M, Yekaninejad MS (2020) Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol 88:106903",
"volume": "88",
"year": "2020"
},
{
"DOI": "10.32794/mr11250047",
"author": "RJ Reiter",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "Melatonin Res",
"key": "1096_CR31",
"unstructured": "Reiter RJ, Ma Q, Sharma R (2020) Treatment of ebola and other infectious diseases: melatonin “goes viral.” Melatonin Res 3:43–57",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04632-4",
"author": "M Rodríguez-Rubio",
"doi-asserted-by": "publisher",
"first-page": "699",
"journal-title": "Trials",
"key": "1096_CR32",
"unstructured": "Rodríguez-Rubio M, Figueira JC, Acuña-Castroviejo D, Borobia AM, Escames G, de la Oliva P (2020) A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial. Trials 21:699",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.arcmed.2018.12.004",
"author": "AL Sánchez-López",
"doi-asserted-by": "publisher",
"first-page": "391",
"journal-title": "Arch Med Res",
"key": "1096_CR33",
"unstructured": "Sánchez-López AL, Ortiz GG, Pacheco-Moises FP, Mireles-Ramírez MA, Bitzer-Quintero OK, Delgado-Lara DLC, Ramírez-Jirano LJ, Velázquez-Brizuela IE (2018) Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch Med Res 49:391–398",
"volume": "49",
"year": "2018"
},
{
"DOI": "10.1016/j.eclinm.2021.100763",
"author": "D Seely",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "1096_CR34",
"unstructured": "Seely D, Legacy M, Auer RC, Fazekas A, Delic E, Anstee C, Angka L, Kennedy MA, Tai LH, Zhang T, Maziak DE, Shamji FM, Sundaresan RS, Gilbert S, Villeneuve PJ, Ashrafi AS, Inculet R, Yasufuku K, Waddell TK, Finley C, Shargall Y, Plourde M, Fergusson DA, Ramsay T, Seely AJE (2021) Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): a randomized placebo controlled clinical trial. EClinicalMedicine 33:100763",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2021.110722",
"author": "A Sen",
"doi-asserted-by": "publisher",
"journal-title": "Med Hypotheses",
"key": "1096_CR35",
"unstructured": "Sen A (2021) Deficient synthesis of melatonin in COVID-19 can impair the resistance of coronavirus patients to mucormycosis. Med Hypotheses 158:110722",
"volume": "158",
"year": "2021"
},
{
"DOI": "10.1034/j.1600-079X.2003.00068.x",
"author": "SY Shiu",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "J Pineal Res",
"key": "1096_CR36",
"unstructured": "Shiu SY, Reiter RJ, Tan DX, Pang SF (2003) Urgent search for safe and effective treatments of severe acute respiratory syndrome: is melatonin a promising candidate drug? J Pineal Res 35:69–70",
"volume": "35",
"year": "2003"
},
{
"DOI": "10.1080/08830185.2020.1756284",
"author": "A Shneider",
"doi-asserted-by": "publisher",
"first-page": "153",
"journal-title": "Int Rev Immunol",
"key": "1096_CR37",
"unstructured": "Shneider A, Kudriavtsev A, Vakhrusheva A (2020) Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol 39:153–162",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1111/j.1600-079X.2007.00535.x",
"author": "PH Wirtz",
"doi-asserted-by": "publisher",
"first-page": "358",
"journal-title": "J Pineal Res",
"key": "1096_CR38",
"unstructured": "Wirtz PH, Bärtschi C, Spillmann M, Ehlert U, von Känel R (2008) Effect of oral melatonin on the procoagulant response to acute psychosocial stress in healthy men: a randomized placebo-controlled study. J Pineal Res 44:358–365",
"volume": "44",
"year": "2008"
},
{
"DOI": "10.1016/j.lfs.2020.117583",
"author": "R Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci",
"key": "1096_CR39",
"unstructured": "Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, Reiter RJ (2020) COVID-19: melatonin as a potential adjuvant treatment. Life Sci 250:117583",
"volume": "250",
"year": "2020"
},
{
"DOI": "10.1111/jpi.12322",
"author": "Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "405",
"journal-title": "J Pineal Res",
"key": "1096_CR40",
"unstructured": "Zhang Y, Li X, Grailer JJ, Wang N, Wang M, Yao J, Zhong R, Gao GF, Ward PA, Tan DX, Li X (2016) Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res 60:405–414",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.1111/bph.14116",
"author": "Y Zisapel",
"doi-asserted-by": "publisher",
"first-page": "3190",
"journal-title": "Br J Pharmacol",
"key": "1096_CR41",
"unstructured": "Zisapel Y (2018) New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol 175:3190–3199",
"volume": "175",
"year": "2018"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s10787-022-01096-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology",
"Immunology"
],
"subtitle": [],
"title": "Efficacy and safety of oral melatonin in patients with severe COVID-19: a randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}