Tocilizumab as a targeted immunomodulatory therapy in the management of severe respiratory illnesses: a multicenter cohort study of COVID-19 patients
et al., Scientific Reports, doi:10.1038/s41598-025-08638-3, Jul 2025
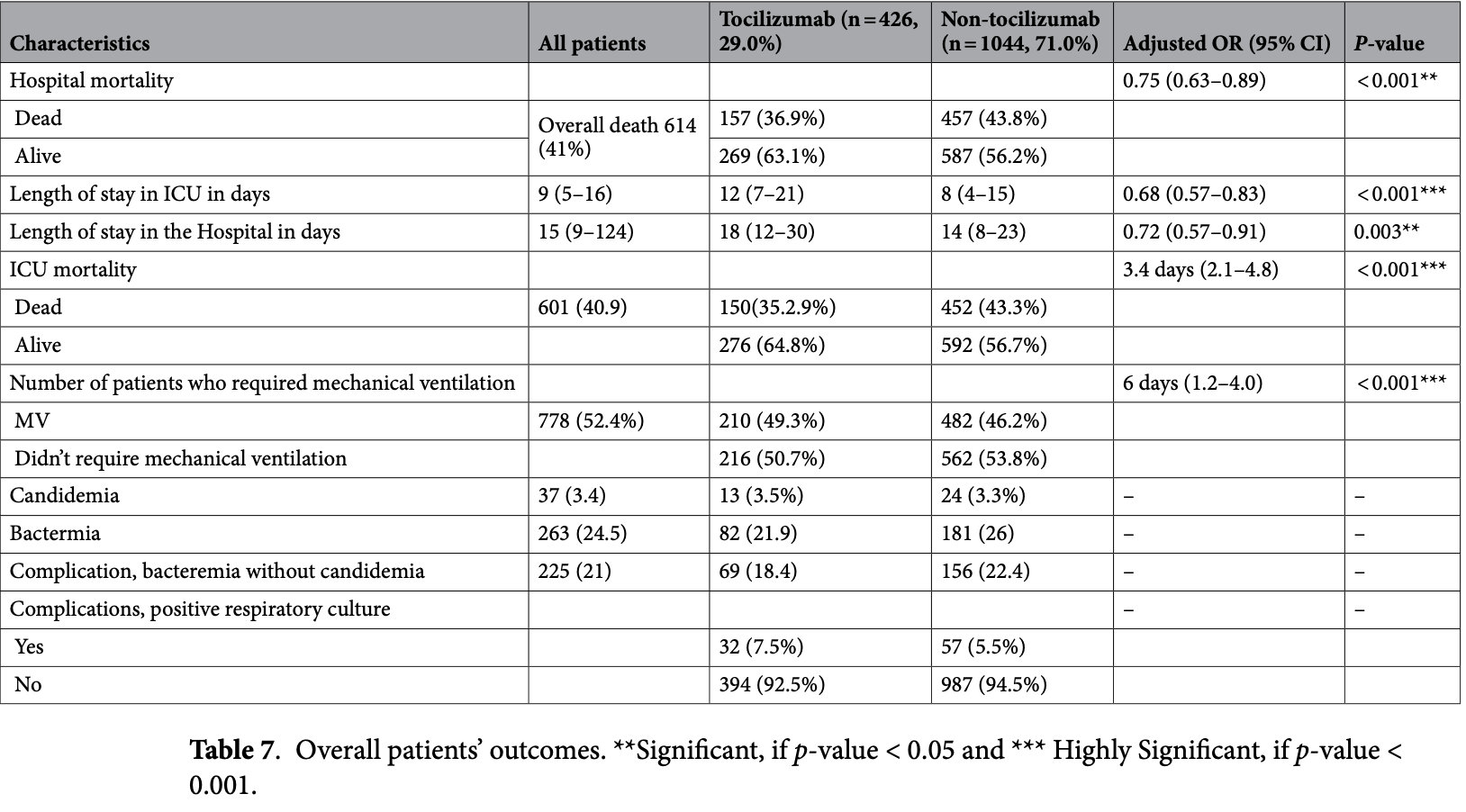
Retrospective 1,470 critically ill COVID-19 patients in Saudi Arabia showing significantly lower mortality with tocilizumab treatment.
|
risk of death, 15.8% lower, RR 0.84, p = 0.002, treatment 157 of 426 (36.9%), control 457 of 1,044 (43.8%), NNT 14, adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Al Mutair et al., 6 Jul 2025, retrospective, Saudi Arabia, peer-reviewed, mean age 55.9, 12 authors, study period 1 March, 2020 - 30 October, 2020.
Contact: muhammad.daniyal@almoosahealth.com.sa.
Tocilizumab as a targeted immunomodulatory therapy in the management of severe respiratory illnesses: a multicenter cohort study of COVID-19 patients
Scientific Reports, doi:10.1038/s41598-025-08638-3
Respiratory pandemics like COVID-19 continued to strain healthcare systems worldwide. Numerous antiviral, antimalarial, and anti-inflammatory treatments were administered to many patients to pursue effective therapeutics. This study aims to assess the efficacy of the anti-inflammatory agent tocilizumab for critically ill patients, specifically in managing respiratory illnesses. This multi-center cohort study included laboratory-confirmed SARS-CoV-2 patients as special cases admitted to the intensive care units (ICUs) of 15 hospitals across Saudi Arabia between March 1, 2020, and October 30, 2020. A total of 1470 critically ill patients with SARS-CoV-2 were included. The study included 1470 patients with a mean age of 55.9 ± 15.1 years; 1088 (74.0%) were male and 382 (26.0%) female. Among them, 29% received Tocilizumab, while 71% received other treatments such as remdesivir, hydroxychloroquine, corticosteroids, convalescent plasma, intravenous immunoglobulin, and plasmapheresis. The median Sequential Organ Failure Assessment (SOFA) score for the cohort was 5 [IQR 3-8], with lower scores observed in the Tocilizumab administered group (p = 0.143). ICU mortality was significantly lower in the tocilizumab group: 150/426 (35.2%) versus 457/1044 (43.8%), p = 0.004. The median length of ICU stay was longer in the Tocilizumab group (12 days; IQR 7-21) than in the non-Tocilizumab group (8 days; IQR 4-15). However, Tocilizumab use was associated with a reduced likelihood of prolonged ICU stay (adjusted OR 0.68; 95% CI 0.57-0.83; p < 0.001). Similarly, the median hospital stay was longer among Tocilizumab recipients (18 days; IQR 12-30) compared to non-recipients (14 days; IQR 8-23). Despite the longer duration, Tocilizumab was associated with a decreased likelihood of extended hospital stay (adjusted OR 0.72; 95% CI 0.57-0.91; p = 0.003). These findings support the beneficial role of Tocilizumab in managing acute respiratory illness due to COVID-19. This study suggests that tocilizumab, particularly when administered early, is associated with reduced mortality and improved outcomes in critically ill patients. These findings not only support the use of tocilizumab as a therapeutic option for severe cases of COVID-19 but also highlight its potential for future respiratory pandemics. Early intervention with tocilizumab may lead to significant benefits, offering a valuable treatment strategy with manageable adverse effects for critically ill patients in future global health crises.
Author contributions Conceptualization: AAM, SA, GYA, MD. Methodology: AAM, SA, GYA, AAR, Software: AAM, SA, GYA, And AAM, MD. Validation: ABM, SA, GYA, AAR, And Formal Analysis: ABM, SA, MD, HA, MD, KA, Investigation: ABM, SA, MD, RM, Data Curation: AAM, SA, MD, KA, AAO, SA, HA, AMA, BA, Writing-Original and Revised Draft: AAM, SA, GYA, AAR, SA, HA, AMA, BA, MD, KA, AAO, RM, Supervision: AAM. Project Administration: AAM. Funding Acquisition: AAM, SA, and GYA.All authors have read and agreed to the published version of the manuscript.
Declarations
Competing interests The authors declare no competing interests.
Ethics approval and consent to participate The informed consent was obtained from each individual participating in the study on an electronic consent form. This study was approved by the Central Institutional Review Board at the Saudi Ministry of Health [H-01-R-009] and by individual centers' ethics boards.
Additional information Correspondence and requests for materials should be addressed to M.D.
References
Al Mutair, Clinical, epidemiological, and laboratory characteristics of mild-to-moderate COVID-19 patients in Saudi Arabia: An observational cohort study, Eur. J. Med. Res
Al-Omari, Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: A descriptive crosssectional study, J. Infect. Public Health
Alattar, Tocilizumab for the treatment of severe coronavirus disease 2019, J. Med. Virol
Albahrani, A case series of severe hospitalized COVID-19 patients treated with tocilizumab and glucocorticoids: A report from Saudi arabian hospital, J. Epidemiol. Glob. Health
Alhazzani, The Saudi critical care society clinical practice guidelines on the management of COVID-19 patients in the intensive care unit, Saudi Crit. Care J
Aljishi, Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia, J. Infect. Public Health
Barry, Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area, J. Epidemiol. Glob. Health
Biran, Tocilizumab among patients with COVID-19 in the intensive care unit: A multicentre observational study, The Lancet Rheumatol
Chen, Chao, Lai, Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients, J. Infect
Dabbous, Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study, Adv. Virol
Desai, Predictors of mortality amongst tocilizumab administered COVID-19 Asian Indians: A predictive study from a tertiary care centre, Cureus
Doi, A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19, Antimicrob. Agents Chemother
Fernández-Ruiz, Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study, J. Med. Virol
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc. Jpn. Acad. Ser. B
Guaraldi, Tocilizumab in patients with severe COVID-19: A retrospective cohort study, Lancet Rheumatol
Ip, Hydroxychloroquine and tocilizumab therapy in COVID-19 patients: An observational study, PLoS ONE
Lang, A current review of COVID-19 for the cardiovascular specialist, Am. Heart J
Mehta, COVID-19: Consider cytokine storm syndromes and immunosuppression, The Lancet
Moore, June, Cytokine release syndrome in severe COVID-19, Science
Obeid, SARS-CoV-2 genetic diversity and variants of concern in Saudi Arabia, J. Infect. Dev. Ctries
Rosas, Tocilizumab in hospitalized patients with severe Covid-19 pneumonia, N. Engl. J. Med
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review, JAMA
Somers, Tocilizumab for treatment of mechanically ventilated patients with COVID-19, Clin. Infect. Dis
Teachey, Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy, Blood J. Am. Soc. Hematol
Zhou, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
DOI record:
{
"DOI": "10.1038/s41598-025-08638-3",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-025-08638-3",
"alternative-id": [
"8638"
],
"article-number": "24120",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "23 January 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "23 June 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "6 July 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no competing interests."
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The informed consent was obtained from each individual participating in the study on an electronic consent form. This study was approved by the Central Institutional Review Board at the Saudi Ministry of Health [H-01-R-009] and by individual centers’ ethics boards."
}
],
"author": [
{
"affiliation": [],
"family": "Al Mutair",
"given": "Abbas",
"sequence": "first"
},
{
"affiliation": [],
"family": "Alhumaid",
"given": "Saad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmad",
"given": "Gasmelseed Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rabaan",
"given": "Ali A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alkubati",
"given": "Sameer A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albaqawi",
"given": "Hamdan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alrasheeday",
"given": "Awatif M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alshammari",
"given": "Bushra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alsaleh",
"given": "Kawther",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mottershead",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daniyal",
"given": "Muhammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Omari",
"given": "Awad",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
7,
6
]
],
"date-time": "2025-07-06T08:50:40Z",
"timestamp": 1751791840000
},
"deposited": {
"date-parts": [
[
2025,
7,
6
]
],
"date-time": "2025-07-06T08:50:41Z",
"timestamp": 1751791841000
},
"indexed": {
"date-parts": [
[
2025,
7,
6
]
],
"date-time": "2025-07-06T09:10:05Z",
"timestamp": 1751793005175,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
7,
6
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
6
]
],
"date-time": "2025-07-06T00:00:00Z",
"timestamp": 1751760000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
6
]
],
"date-time": "2025-07-06T00:00:00Z",
"timestamp": 1751760000000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-025-08638-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-08638-3",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-08638-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2025,
7,
6
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
6
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41586-020-2012-7",
"author": "P Zhou",
"doi-asserted-by": "publisher",
"first-page": "270",
"issue": "7798",
"journal-title": "Nature",
"key": "8638_CR1",
"unstructured": "Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798), 270–273 (2020).",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0695-z",
"doi-asserted-by": "crossref",
"key": "8638_CR2",
"unstructured": "The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 4: 536–544 (2020)."
},
{
"DOI": "10.4103/sccj.sccj_15_20",
"author": "W Alhazzani",
"doi-asserted-by": "publisher",
"first-page": "27",
"issue": "2",
"journal-title": "Saudi Crit. Care J.",
"key": "8638_CR3",
"unstructured": "Alhazzani, W. et al. The Saudi critical care society clinical practice guidelines on the management of COVID-19 patients in the intensive care unit. Saudi Crit. Care J. 4(2), 27–44 (2020).",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1016/j.jiph.2020.09.003",
"author": "A Al-Omari",
"doi-asserted-by": "publisher",
"first-page": "1639",
"issue": "11",
"journal-title": "J. Infect. Public Health",
"key": "8638_CR4",
"unstructured": "Al-Omari, A. et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: A descriptive cross-sectional study. J. Infect. Public Health 13(11), 1639–1644 (2020).",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1186/s40001-020-00462-x",
"author": "A Al Mutair",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Eur. J. Med. Res.",
"key": "8638_CR5",
"unstructured": "Al Mutair, A. et al. Clinical, epidemiological, and laboratory characteristics of mild-to-moderate COVID-19 patients in Saudi Arabia: An observational cohort study. Eur. J. Med. Res. 25, 1–8 (2020).",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1016/j.jiph.2020.11.002",
"author": "JM AlJishi",
"doi-asserted-by": "publisher",
"first-page": "6",
"issue": "1",
"journal-title": "J. Infect. Public Health",
"key": "8638_CR6",
"unstructured": "AlJishi, J. M. et al. Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J. Infect. Public Health 14(1), 6–11 (2021).",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.2991/jegh.k.200806.002",
"author": "M Barry",
"doi-asserted-by": "publisher",
"first-page": "214",
"issue": "3",
"journal-title": "J. Epidemiol. Glob. Health",
"key": "8638_CR7",
"unstructured": "Barry, M. et al. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J. Epidemiol. Glob. Health 10(3), 214–221 (2020).",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.2991/jegh.k.210112.001",
"author": "S AlBahrani",
"doi-asserted-by": "publisher",
"first-page": "233",
"issue": "2",
"journal-title": "J. Epidemiol. Glob. Health",
"key": "8638_CR8",
"unstructured": "AlBahrani, S. et al. A case series of severe hospitalized COVID-19 patients treated with tocilizumab and glucocorticoids: A report from Saudi arabian hospital. J. Epidemiol. Glob. Health 11(2), 233–237 (2021).",
"volume": "11",
"year": "2021"
},
{
"author": "DT Teachey",
"first-page": "5154",
"issue": "26",
"journal-title": "Blood J. Am. Soc. Hematol.",
"key": "8638_CR9",
"unstructured": "Teachey, D. T. et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood J. Am. Soc. Hematol. 121(26), 5154–5157 (2013).",
"volume": "121",
"year": "2013"
},
{
"DOI": "10.1126/science.abb8925",
"author": "JB Moore",
"doi-asserted-by": "publisher",
"first-page": "473",
"issue": "6490",
"journal-title": "Science",
"key": "8638_CR10",
"unstructured": "Moore, J. B. & June, C. H. Cytokine release syndrome in severe COVID-19. Science 368(6490), 473–4 (2020).",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "10229",
"journal-title": "The Lancet",
"key": "8638_CR11",
"unstructured": "Mehta, P. et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet 395(10229), 1033–1034 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30173-9",
"author": "G Guaraldi",
"doi-asserted-by": "publisher",
"first-page": "e474",
"issue": "8",
"journal-title": "Lancet Rheumatol.",
"key": "8638_CR12",
"unstructured": "Guaraldi, G. et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2(8), e474–e484 (2020).",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0237693",
"author": "A Ip",
"doi-asserted-by": "publisher",
"first-page": "e0237693",
"issue": "8",
"journal-title": "PLoS ONE",
"key": "8638_CR13",
"unstructured": "Ip, A. et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients: An observational study. PLoS ONE 15(8), e0237693 (2020).",
"volume": "15",
"year": "2020"
},
{
"author": "JM Sanders",
"first-page": "1824",
"issue": "18",
"journal-title": "JAMA",
"key": "8638_CR14",
"unstructured": "Sanders, J. M., Monogue, M. L., Jodlowski, T. Z. & Cutrell, J. B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA 323(18), 1824–1836 (2020).",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa954",
"author": "EC Somers",
"doi-asserted-by": "publisher",
"first-page": "e445",
"issue": "2",
"journal-title": "Clin. Infect. Dis.",
"key": "8638_CR15",
"unstructured": "Somers, E. C. et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 73(2), e445–e454 (2021).",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1002/jmv.25964",
"author": "R Alattar",
"doi-asserted-by": "publisher",
"first-page": "2042",
"issue": "10",
"journal-title": "J. Med. Virol.",
"key": "8638_CR16",
"unstructured": "Alattar, R. et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 92(10), 2042–2049 (2020).",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.3855/jidc.15350",
"author": "DA Obeid",
"doi-asserted-by": "publisher",
"first-page": "1782",
"issue": "12",
"journal-title": "J. Infect. Dev. Ctries.",
"key": "8638_CR17",
"unstructured": "Obeid, D. A. et al. SARS-CoV-2 genetic diversity and variants of concern in Saudi Arabia. J. Infect. Dev. Ctries. 15(12), 1782–1791 (2021).",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/j.ahj.2020.04.025",
"author": "JP Lang",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "Am. Heart J.",
"key": "8638_CR18",
"unstructured": "Lang, J. P. et al. A current review of COVID-19 for the cardiovascular specialist. Am. Heart J. 226, 29–44 (2020).",
"volume": "226",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "10285",
"journal-title": "Lancet (London, England)",
"key": "8638_CR19",
"unstructured": "RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (London, England) 397(10285), 1637 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028700",
"author": "IO Rosas",
"doi-asserted-by": "publisher",
"first-page": "1503",
"issue": "16",
"journal-title": "N. Engl. J. Med.",
"key": "8638_CR20",
"unstructured": "Rosas, I. O. et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 384(16), 1503–1516 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.2183/pjab.93.027",
"author": "Y Furuta",
"doi-asserted-by": "publisher",
"first-page": "449",
"issue": "7",
"journal-title": "Proc. Jpn. Acad. Ser. B",
"key": "8638_CR21",
"unstructured": "Furuta, Y., Komeno, T. & Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 93(7), 449–463 (2017).",
"volume": "93",
"year": "2017"
},
{
"author": "HM Dabbous",
"first-page": "949",
"journal-title": "Adv. Virol.",
"key": "8638_CR22",
"unstructured": "Dabbous, H. M. et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Adv. Virol. 166, 949–954 (2021).",
"volume": "166",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2020.12.005",
"author": "PJ Chen",
"doi-asserted-by": "publisher",
"first-page": "186",
"issue": "5",
"journal-title": "J. Infect.",
"key": "8638_CR23",
"unstructured": "Chen, P. J., Chao, C. M. & Lai, C. C. Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients. J. Infect. 82(5), 186 (2020).",
"volume": "82",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01897-20",
"author": "Y Doi",
"doi-asserted-by": "publisher",
"first-page": "10",
"issue": "12",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "8638_CR24",
"unstructured": "Doi, Y. et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob. Agents Chemother. 64(12), 10–128 (2020).",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26308",
"author": "M Fernández-Ruiz",
"doi-asserted-by": "publisher",
"first-page": "831",
"issue": "2",
"journal-title": "J. Med. Virol.",
"key": "8638_CR25",
"unstructured": "Fernández-Ruiz, M. et al. Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study. J. Med. Virol. 93(2), 831–842 (2021).",
"volume": "93",
"year": "2021"
},
{
"author": "HD Desai",
"first-page": "e13116",
"issue": "2",
"journal-title": "Cureus",
"key": "8638_CR26",
"unstructured": "Desai, H. D. et al. Predictors of mortality amongst tocilizumab administered COVID-19 Asian Indians: A predictive study from a tertiary care centre. Cureus 13(2), e13116 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(20)30277-0",
"author": "N Biran",
"doi-asserted-by": "publisher",
"first-page": "e603",
"issue": "10",
"journal-title": "The Lancet Rheumatol.",
"key": "8638_CR27",
"unstructured": "Biran, N. et al. Tocilizumab among patients with COVID-19 in the intensive care unit: A multicentre observational study. The Lancet Rheumatol. 2(10), e603–e612 (2020).",
"volume": "2",
"year": "2020"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-025-08638-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tocilizumab as a targeted immunomodulatory therapy in the management of severe respiratory illnesses: a multicenter cohort study of COVID-19 patients",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "15"
}