Add-on inhaled budesonide in the treatment of hospitalised patients with COVID-19: a randomised clinical trial
et al., European Respiratory Journal, doi:10.1183/13993003.03036-2021, TACTIC, NCT04355637, Feb 2022
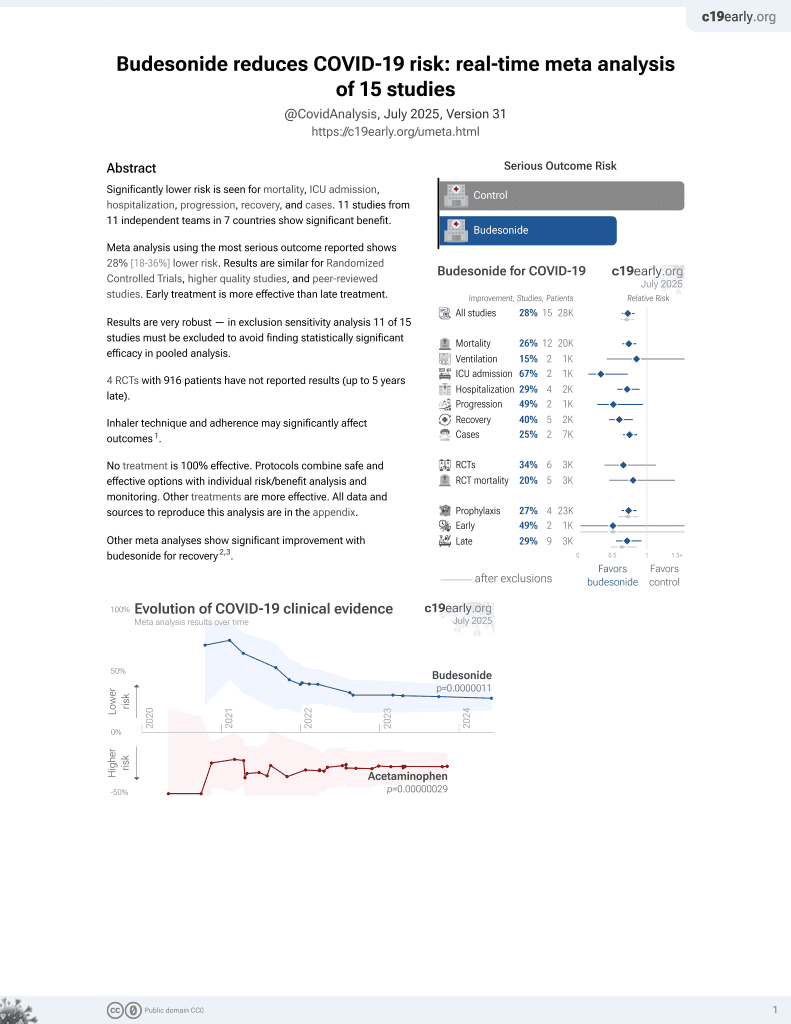
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small early-terminated RCT with 40 inhaled budesonide and 49 control patients, showing no significant differences. 400µg/12h via Pulmicort Turbuhaler.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
|
risk of death, 22.5% higher, RR 1.23, p = 1.00, treatment 1 of 40 (2.5%), control 1 of 49 (2.0%), day 90.
|
|
risk of progression, 38.7% lower, RR 0.61, p = 0.69, treatment 2 of 40 (5.0%), control 4 of 49 (8.2%), NNT 32.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Agustí et al., 10 Feb 2022, Randomized Controlled Trial, Spain, peer-reviewed, 21 authors, study period 21 April, 2020 - 16 March, 2021, trial NCT04355637 (history) (TACTIC).
Abstract: Early View
Research letter
Add-on inhaled budesonide in the treatment of
hospitalised patients with COVID-19: a
randomised clinical trial
Alvar Agustí, Gaston De Stefano, Alberto Levi, Xavier Muñoz, Christian Romero-Mesones, Oriol Sibila,
Alejandra Lopez-Giraldo, Vicente Plaza Moral, Elena Curto, Andrés L. Echazarreta, Silvana E.
Márquez, Sergi Pascual-Guàrdia, Salud Santos, Alicia Marin, Luis Valdés, Fernando Saldarini, Clara
Salgado, Georgina Casanovas, Sara Varea, José Ríos, Rosa Faner
Please cite this article as: Agustí A, De Stefano G, Levi A, et al. Add-on inhaled budesonide in
the treatment of hospitalised patients with COVID-19: a randomised clinical trial. Eur Respir J
2022; in press (https://doi.org/10.1183/13993003.03036-2021).
This manuscript has recently been accepted for publication in the European Respiratory Journal. It is
published here in its accepted form prior to copyediting and typesetting by our production team. After
these production processes are complete and the authors have approved the resulting proofs, the article
will move to the latest issue of the ERJ online.
Copyright ©The authors 2022. This version is distributed under the terms of the Creative Commons
Attribution Non-Commercial Licence 4.0. For commercial reproduction rights and permissions contact
permissions@ersnet.org
January 20, 2022
Research letter ERJ-03036-2021
SECOND REVISION
ADD-ON INHALED BUDESONIDE IN THE TREATMENT OF
HOSPITALIZED PATIENTS WITH COVID-19:
A RANDOMIZED CLINICAL TRIAL
Alvar Agustí1-4, Gaston De Stefano5, Alberto Levi5, Xavier Muñoz4,6,7,
Christian Romero-Mesones4,6, Oriol Sibila1-4, Alejandra Lopez-Giraldo2-4,
Vicente Plaza Moral4,8-10, Elena Curto4,8-10, Andrés L. Echazarreta11,
Silvana E. Márquez11, Sergi Pascual-Guàrdia4,12,13, Salud Santos2,4,14,15,
Alicia Marin4,16, Luis Valdés17-19, Fernando Saldarini20, Clara Salgado21,
Georgina Casanovas1,3, Sara Varea1,3, José Ríos1,3,7, Rosa Faner2-4
1. Hospital Clinic, Barcelona, Spain.
2. Universitat Barcelona, Spain.
3. Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS),
Barcelona, Spain.
4. CIBER Enfermedades Respiratorias, Spain.
5. Servicio de Neumotisiologia, Hospital Francisco Muñiz, Buenos Aires,
Argentina
6. Servei Pneumologia H. Vall d’Hebron, Barcelona Spain.
7. Biostatistics Unit, Faculty of Medicine, Universitat Autònoma de Barcelona
8. Department of Respiratory Medicine, Hospital de la Santa Creu i Sant Pau.
Barcelona, Spain
9. Institut d’Investigació Biomédica Sant Pau (IIB Sant Pau). Barcelona, Spain
10. Universitat Autònoma de Barcelona, Department of Medicine, Barcelona, Spain
11. Servicio de Neumonología, Hospital San Juan de Dios de La Plata, Buenos
Aires, Argentina
12. Servei de Pneumologia, Hospital del Mar - IMIM. Barcelona, Spain
13. Universitat Pompeu Fabra. Barcelona, Spain
14. Department of Pulmonary Medicine, Bellvitge University Hospital, L’Hospitalet
de Llobregat, Barcelona, Spain
15. Institut d’Investigació Biomèdica de Bellvitge – IDIBELL, Spain
16. Hospital Universitari Germans Trias i Pujol, Badalona, Spain
17. Servicio de Neumología. Complejo Hospitalario Universitario de Santiago,
Santiago de Compostela, Spain
18. Instituto de Investigaciones Sanitarias (IDIS), Santiago de Compostela, Spain
19. Universidad de Santiago de Compostela, Spain
20. Sección de Neumotisiologia. Hospital Donación Francisco Santojanni, Buenos
Aires, Argentina.
21. Centro de Educación Médica e Investigaciones Clínicas Norberto Quirno
Correspondence:..
DOI record:
{
"DOI": "10.1183/13993003.03036-2021",
"ISSN": [
"0903-1936",
"1399-3003"
],
"URL": "http://dx.doi.org/10.1183/13993003.03036-2021",
"alternative-id": [
"10.1183/13993003.03036-2021"
],
"author": [
{
"affiliation": [],
"family": "Agustí",
"given": "Alvar",
"sequence": "first"
},
{
"affiliation": [],
"family": "De Stefano",
"given": "Gaston",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levi",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero-Mesones",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sibila",
"given": "Oriol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez-Giraldo",
"given": "Alejandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moral",
"given": "Vicente Plaza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curto",
"given": "Elena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4997-5768",
"affiliation": [],
"authenticated-orcid": false,
"family": "Echazarreta",
"given": "Andrés L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Márquez",
"given": "Silvana E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pascual-Guàrdia",
"given": "Sergi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Salud",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9358-2120",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marin",
"given": "Alicia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valdés",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saldarini",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salgado",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casanovas",
"given": "Georgina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Varea",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ríos",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faner",
"given": "Rosa",
"sequence": "additional"
}
],
"container-title": [
"European Respiratory Journal"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ersjournals.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T19:10:14Z",
"timestamp": 1644520214000
},
"deposited": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T19:10:20Z",
"timestamp": 1644520220000
},
"funder": [
{
"DOI": "10.13039/100004325",
"doi-asserted-by": "publisher",
"name": "AstraZeneca"
}
],
"indexed": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T19:42:28Z",
"timestamp": 1644522148010
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0903-1936"
},
{
"type": "electronic",
"value": "1399-3003"
}
],
"issued": {
"date-parts": [
[
2022,
2,
10
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T00:00:00Z",
"timestamp": 1644451200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1183/13993003.03036-2021",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "81",
"original-title": [],
"page": "2103036",
"prefix": "10.1183",
"published": {
"date-parts": [
[
2022,
2,
10
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
10
]
]
},
"publisher": "European Respiratory Society (ERS)",
"reference": [
{
"DOI": "10.1056/NEJMoa2110362",
"doi-asserted-by": "crossref",
"key": "2022021011100763000_13993003.03036-2021v1.1",
"unstructured": "Thompson MG , Stenehjem E , Grannis S , et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J Med 2021."
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "crossref",
"key": "2022021011100763000_13993003.03036-2021v1.2",
"unstructured": "Polack FP , Thomas SJ , Kitchin N , et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020."
},
{
"DOI": "10.1056/NEJMoa2110362",
"doi-asserted-by": "publisher",
"key": "2022021011100763000_13993003.03036-2021v1.3"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2022021011100763000_13993003.03036-2021v1.4"
},
{
"key": "2022021011100763000_13993003.03036-2021v1.5",
"unstructured": "Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. New England Journal of Medicine, 2020."
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"doi-asserted-by": "publisher",
"key": "2022021011100763000_13993003.03036-2021v1.6"
},
{
"key": "2022021011100763000_13993003.03036-2021v1.7",
"unstructured": "Group TWREAfC-TW . Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020."
},
{
"key": "2022021011100763000_13993003.03036-2021v1.8",
"unstructured": "Ramakrishnan S , Nicolau DV , Langford B , et al. Inhaled budesonide in the treatment of early COVID-19 illness: a randomised controlled trial. Lancet Respir Med 2021 (in press)."
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"doi-asserted-by": "publisher",
"key": "2022021011100763000_13993003.03036-2021v1.9"
},
{
"DOI": "10.1016/S2213-2600(21)00171-5",
"article-title": "Early treatment with inhaled budesonide to prevent clinical deterioration in patients with COVID-19",
"author": "Agusti",
"doi-asserted-by": "crossref",
"first-page": "682",
"journal-title": "Lancet Respir Med",
"key": "2022021011100763000_13993003.03036-2021v1.10",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2021.6759",
"doi-asserted-by": "crossref",
"key": "2022021011100763000_13993003.03036-2021v1.11",
"unstructured": "Clemency BM , Varughese R , Gonzalez-Rojas Y , et al. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA Intern Med 2021."
},
{
"DOI": "10.1038/s41598-020-59732-7",
"article-title": "Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "3044",
"journal-title": "Sci Rep",
"key": "2022021011100763000_13993003.03036-2021v1.12",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "2022021011100763000_13993003.03036-2021v1.13"
},
{
"DOI": "10.1093/oxfordjournals.aje.a114212",
"doi-asserted-by": "publisher",
"key": "2022021011100763000_13993003.03036-2021v1.14"
},
{
"DOI": "10.1093/aje/kwh221",
"doi-asserted-by": "publisher",
"key": "2022021011100763000_13993003.03036-2021v1.15"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"score": 1,
"short-container-title": [
"Eur Respir J"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": [
"Add-on inhaled budesonide in the treatment of hospitalised patients with COVID-19: a randomised clinical trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1183/ers-crossmark-policy"
}