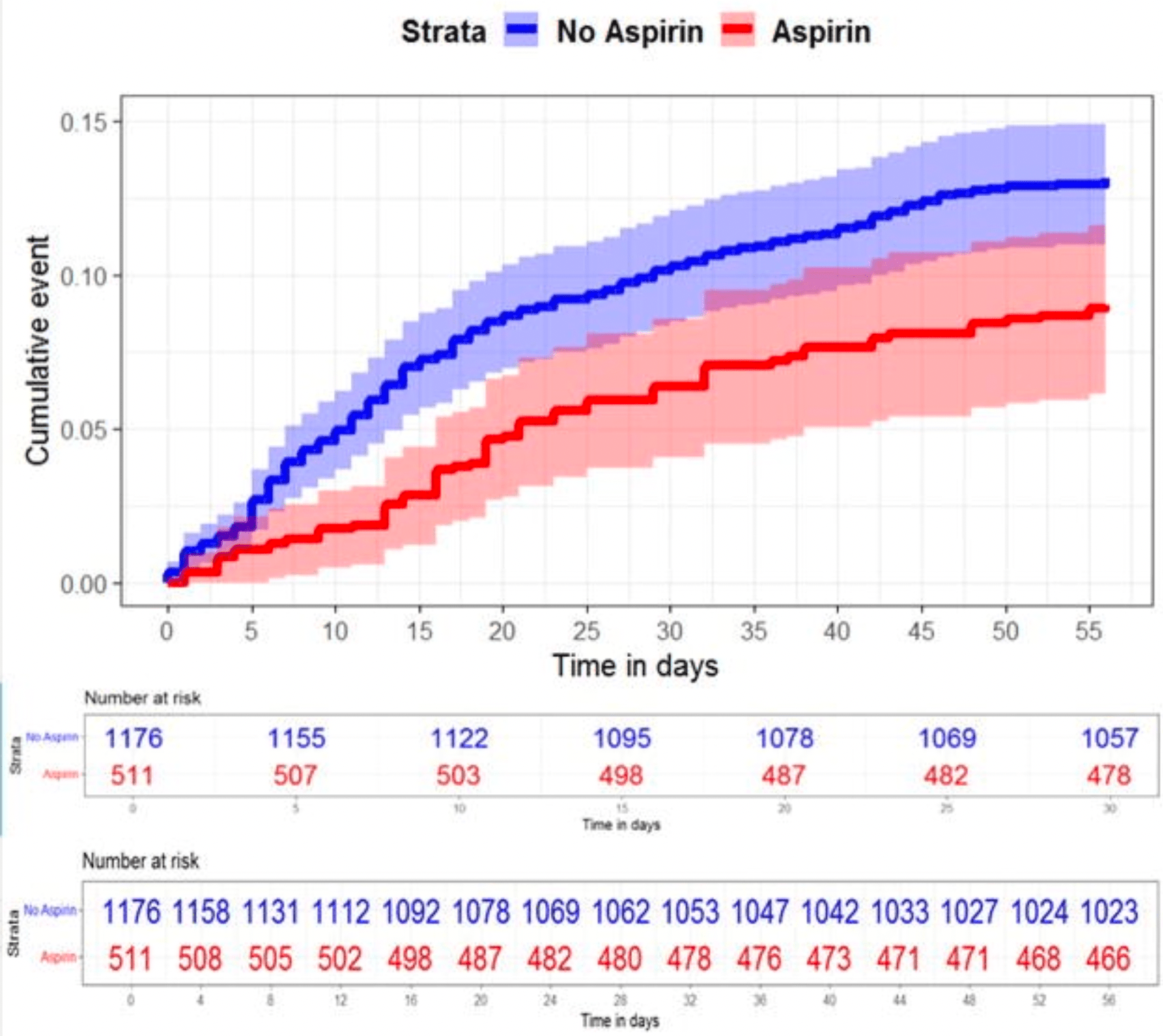
Association of mortality and aspirin use for COVID-19 residents at VA Community Living Center Nursing Homes
et al., medRxiv, doi:10.1101/2022.08.03.22278392, Aug 2022
Retrospective 1,687 nursing home residents in the USA, showing significantly lower risk of mortality with chronic low-dose aspirin use. Low dose 81mg aspirin users had treatment ≥10 of 14 days prior to the positive COVID date, control patients had no aspirin use in the prior 14 days.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 33.0% lower, HR 0.67, p = 0.03, treatment 46 of 511 (9.0%), control 201 of 1,176 (17.1%), Cox proportional hazards, day 56.
|
|
risk of death, 40.0% lower, HR 0.60, p = 0.01, treatment 33 of 511 (6.5%), control 154 of 1,176 (13.1%), Cox proportional hazards, day 30.
|
|
risk of hospitalization, 20.0% lower, HR 0.80, p = 0.13, treatment 103 of 511 (20.2%), control 352 of 1,176 (29.9%), Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Abul et al., 4 Aug 2022, retrospective, USA, preprint, mean age 72.3, 10 authors, study period 13 December, 2020 - 18 September, 2021.
Contact: yasin_abul@brown.edu.
Association of mortality and aspirin use for COVID-19 residents at VA Community Living Center Nursing Homes
doi:10.1101/2022.08.03.22278392
Background/Objectives: Coronavirus disease 2019 (COVID-19) is associated with a hypercoagulable state and increased thrombotic risk in infected individuals. Several complex and varied coagulation abnormalities were proposed for this association 1 .Acetylsalicylic acid(ASA, aspirin) is known to have inflammatory, antithrombotic properties and its use was reported as having potency to reduce RNA synthesis and replication of some types of coronaviruses including human coronavirus-299E (CoV-229E) and Middle East Respiratory Syndrome (MERS)-CoV 2,3 . We hypothesized that chronic low dose aspirin use may decrease COVID-19 mortality relative to ASA non-users. Methods: This is a retrospective, observational cohort analysis of residents residing at Veterans Affairs Community Living Centers from December 13, 2020, to September 18, 2021, with a positive SARS-CoV-2 PCR test. Low dose aspirin users had low dose (81mg) therapy (10 of 14 days) prior to the positive COVID date and were compared to aspirin non-users (no ASA in prior 14 days). The primary outcome was mortality at 30 and 56 days post positive test and hospitalization.
Results: We identified 1.823 residents who had SARS-CoV-2 infection and 1,687 residents were eligible for the study. Aspirin use was independently associated with a reduced risk of 30 days of mortality (adjusted HR, 0.60, 95% CI, 0.40-0.90) and 56 days of mortality (adjusted HR, 0.67, 95% CI, 0.47-0.95) Conclusion: Chronic low dose aspirin use for primary or secondary prevention of cardiovascular events is associated with lower COVID-19 mortality. Although additional randomized controlled trials are required to understand these associations and the potential implications more fully for improving care, aspirin remains a medication with known side effects and clinical practice should not change based on these findings.
References
Alter, Sekaly, Beyond adjuvants: Antagonizing inflammation to enhance vaccine immunity, Vaccine
Aly, Ibrahim, Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy?, Med Hypotheses
Awtry, Loscalzo, None, Aspirin. Circulation
Bianconi, Violi, Fallarino, Pignatelli, Sahebkar et al., Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19 ?, Drugs
Campbell, Smyth, Montalescot, Steinhubl, Aspirin dose for the prevention of cardiovascular disease: a systematic review, JAMA
Capone, Tacconelli, Sciulli, Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects, Circulation
Chambers, Akbar, Can blocking inflammation enhance immunity during aging?, J Allergy Clin Immunol
Chow, Khanna, Kethireddy, Aspirin Use Is Associated With Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients With Coronavirus Disease 2019, Anesth Analg
Chow, Rahnavard, Gomberg-Maitland, Association of Early Aspirin Use With In-Hospital Mortality in Patients With Moderate COVID-19, JAMA Netw Open
Glatthaar-Saalmüller, Mair, Saalmüller, Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study, Influenza Other Respir Viruses
Group, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Hm, Mehta, Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19, Am J Cardiol
Jiménez, García-Sanchez, Rali, Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis, Chest
Kunutsor, Laukkanen, Incidence of venous and arterial thromboembolic complications in COVID-19: A systematic review and meta-analysis, Thromb Res
Li, Li, Wang, Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study, Stroke Vasc Neurol
Liu, Huang, Li, Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19, Medicine
Maier, Truong, Auld, Polly, Tanksley et al., COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia?, Lancet
Martha, Pranata, Lim, Wibowo, Akbar, Active prescription of low-dose aspirin during or prior to hospitalization and mortality in COVID-19: A systematic review and meta-analysis of adjusted effect estimates, Int J Infect Dis
Mazur, Wurzer, Ehrhardt, Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity, Cell Microbiol
Mccarty, Block, Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy, Integr Cancer Ther
Meizlish, Goshua, Liu, Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis, Am J Hematol
Muller, Kn, Ziebuhr, Pleschka, L-lysine acetylsalicylate + glycine impairs coronavirus replication, J Antivir Antiretrovir
Osborne, Veigulis, Arreola, Mahajan, Roosli et al., Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration, PLoS One
Panka, De Grooth, Spoelstra-De Man, Looney, Tuinman, Prevention or Treatment of Ards With Aspirin: A Review of Preclinical Models and Meta-Analysis of Clinical Studies, Shock
Patrono, Ciabattoni, Patrignani, Clinical pharmacology of platelet cyclooxygenase inhibition, Circulation
Sahai, Bhandari, Godwin, Effect of aspirin on short-term outcomes in hospitalized patients with COVID-19, Vasc Med
Trujillo-Murillo, Rincón-Sánchez, Martínez-Rodríguez, Acetylsalicylic acid inhibits C virus RNA and protein expression through cyclooxygenase 2 signaling pathways, Hepatology
Wheat, Irani, Hughes, Josephson, Dolansky, Insights from Monitoring Aspirin Adherence: A Medication Adherence Cascade Tool, Patient Prefer Adherence
Wichmann, Autopsy Findings and Venous Thromboembolism in Patients With COVID-19, Ann Intern Med
Wijaya, Andhika, Huang, Purwiga, Budiman, The effects of aspirin on the outcome of COVID-19: A systematic review and meta-analysis, Clin Epidemiol Glob Health
Wilson, Kvalsvig, Barnard, Baker, Case-Fatality Risk Estimates for COVID-19
Yang, Yu, Xu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med
Yuan, Chen, Li, Chen, Wang et al., Mortality and pre-hospitalization use of lowdose aspirin in COVID-19 patients with coronary artery disease, J Cell Mol Med
DOI record:
{
"DOI": "10.1101/2022.08.03.22278392",
"URL": "http://dx.doi.org/10.1101/2022.08.03.22278392",
"abstract": "<jats:p>Background/Objectives: Coronavirus disease 2019 (COVID-19) is associated with a hypercoagulable state and increased thrombotic risk in infected individuals. Several complex and varied coagulation abnormalities were proposed for this association1 .Acetylsalicylic acid(ASA, aspirin) is known to have inflammatory, antithrombotic properties and its use was reported as having potency to reduce RNA synthesis and replication of some types of coronaviruses including human coronavirus-299E (CoV-229E) and Middle East Respiratory Syndrome (MERS)-CoV 2,3. We hypothesized that chronic low dose aspirin use may decrease COVID-19 mortality relative to ASA non-users. \nMethods: This is a retrospective, observational cohort analysis of residents residing at Veterans Affairs Community Living Centers from December 13, 2020, to September 18, 2021, with a positive SARS-CoV-2 PCR test. Low dose aspirin users had low dose (81mg) therapy (10 of 14 days) prior to the positive COVID date and were compared to aspirin non-users (no ASA in prior 14 days). The primary outcome was mortality at 30 and 56 days post positive test and hospitalization within 14 days of positive test result. \nResults: We identified 1.823 residents who had SARS-CoV-2 infection and 1,687 residents were eligible as a final analytic sample after excluding high dose and intermittent/partial dose aspirin users. Overall mean age was 72.28+/-11.66 years and 3.3% (n=67) female in the final analytic sample. Among the 511 (30.3%) residents taking chronic low dose aspirin, 30-day mortality after an initial SARS-CoV-2 test establishing infection was 6.46% (n=33) compared to 10.29% (n=121) of non-users (SMD >0.1). 56-day mortality after initial SARS-CoV-2 test establishing infection was 9.0% (n=46) compared to 13.18% (n=155) not taking low dose aspirin (SMD >0.1). Cox proportional hazards model showed that aspirin use was independently associated with a reduced risk of 30 days of mortality (adjusted HR, 0.60, 95% CI, 0.40-0.90) and 56 days of mortality (adjusted HR, 0.67, 95% CI, 0.47-0.95)\nConclusion: In this retrospective observational study of VA Community Living Center residents infected with SARS-CoV-2, low dose aspirin use for primary or secondary prevention of cardiovascular events is associated with lower COVID-19 mortality and fewer breakthrough cases. Although additional randomized controlled trials are required to understand these associations and the potential implications more fully for improving care, aspirin remains a medication with known side effects and clinical practice should not change based on these findings.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
8,
4
]
]
},
"author": [
{
"affiliation": [],
"family": "Abul",
"given": "Yasin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Devone",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayer",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Halladay",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McConeghy",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mujahid",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Mriganka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leeder",
"given": "Ciera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gravenstein",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rudolph",
"given": "James L",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
5
]
],
"date-time": "2022-08-05T02:30:10Z",
"timestamp": 1659666610000
},
"deposited": {
"date-parts": [
[
2022,
8,
5
]
],
"date-time": "2022-08-05T02:30:11Z",
"timestamp": 1659666611000
},
"group-title": "Geriatric Medicine",
"indexed": {
"date-parts": [
[
2022,
8,
5
]
],
"date-time": "2022-08-05T03:14:32Z",
"timestamp": 1659669272320
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
4
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.08.03.22278392",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
8,
4
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
8,
4
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.08.03.22278392"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Association of mortality and aspirin use for COVID-19 residents at VA Community Living Center Nursing Homes",
"type": "posted-content"
}