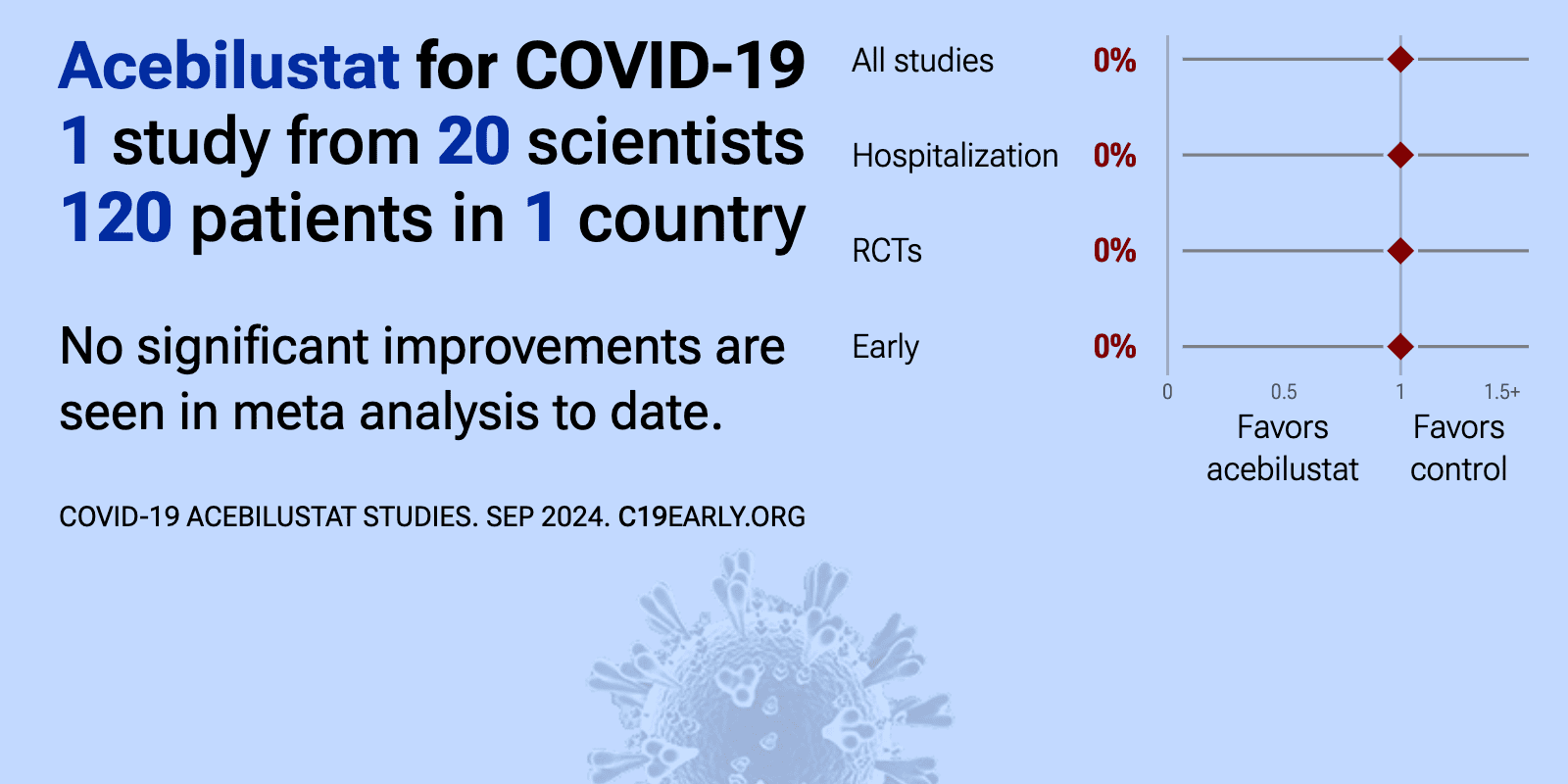
Mar 30 2023 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciad187 | Evaluation of Acebilustat, a Selective Inhibitor of Leukotriene B4 Biosynthesis, for Treatment of Outpatients With Mild-Moderate Coronavirus Disease 2019: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial |
| 80% lower progression (p=0.21), 67% worse recovery (p=0.07), and 6% improved viral clearance. RCT 120 outpatients showing no significant difference in time to sustained symptom resolution or viral clearance with acebilustat treatment. Subgroup analyses showed consistent patterns of longer symptom duration in treated participants. | ||