Prophylactic Administration of the Monoclonal Antibody Adintrevimab Protects against SARS-CoV-2 in Hamster and Non-Human Primate Models of COVID-19
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01353-22, Jan 2023
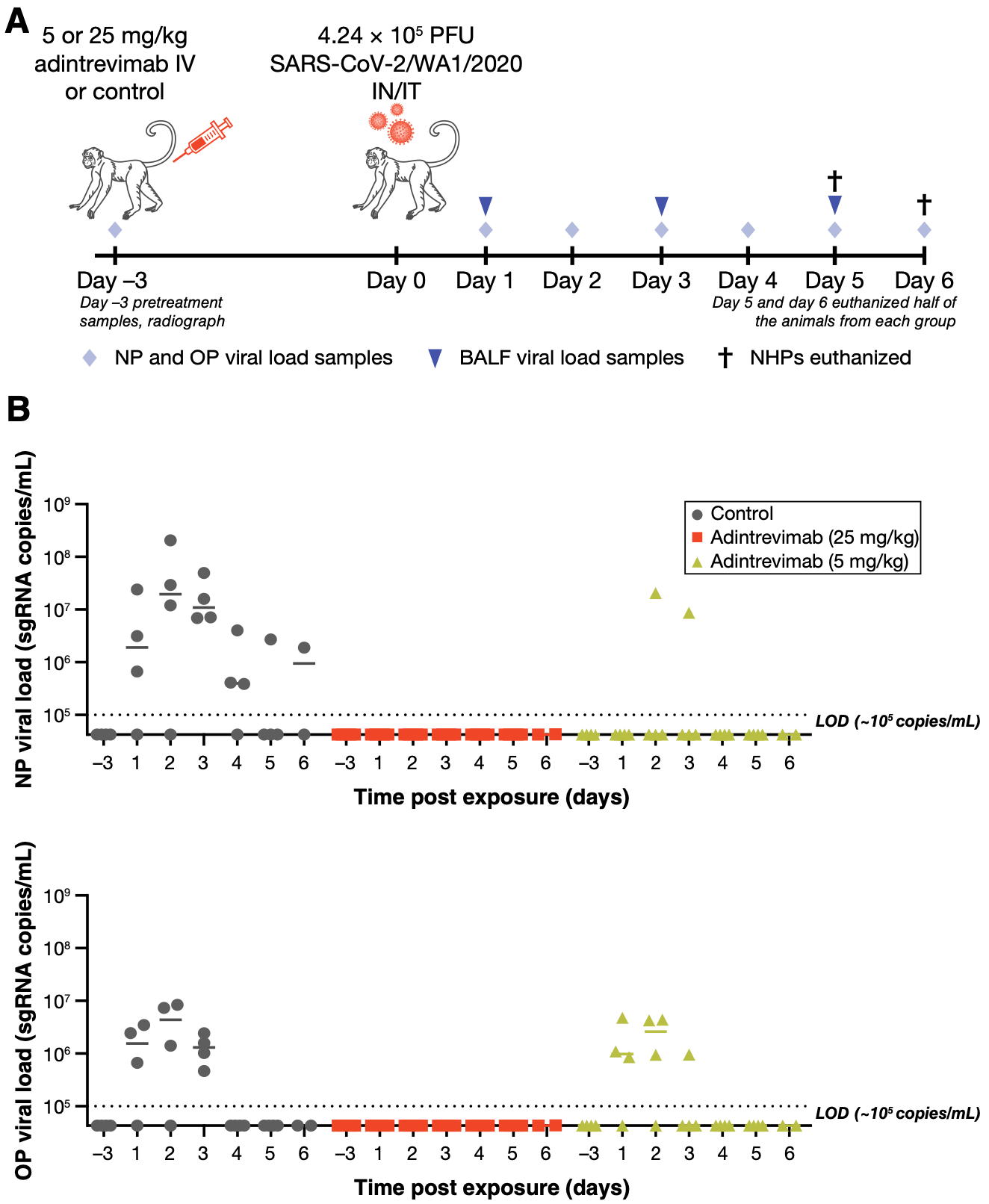
Hamster and monkey study showing dose-dependent protection against SARS-CoV-2 infection with prophylactic administration of a single dose of adintrevimab.
Zumbrun et al., 24 Jan 2023, USA, peer-reviewed, 17 authors.
Contact: knarayan@invivyd.com.
Prophylactic Administration of the Monoclonal Antibody Adintrevimab Protects against SARS-CoV-2 in Hamster and Non-Human Primate Models of COVID-19
Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01353-22
Adintrevimab is a human immunoglobulin G1 monoclonal antibody engineered to have broad neutralization against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and other SARS-like coronaviruses with pandemic potential. In both Syrian golden hamster and rhesus macaque models, prophylactic administration of a single dose of adintrevimab provided protection against SARS-CoV-2/WA1/2020 infection in a dose-dependent manner, as measured by significant reductions in lung viral load and virus-induced lung pathology, and by inhibition of viral replication in the upper and lower respiratory tract.
References
Andrews, Stowe, Kirsebom, Toffa, Rickeard et al., COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant, N Engl J Med, doi:10.1056/NEJMoa2119451
Dejnirattisai, Huo, Zhou, Zahradník, Supasa et al., SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses, doi:10.1016/j.cell.2021.12.046
Dejnirattisai, Zhou, Supasa, Liu, Mentzer et al., Antibody evasion by the P.1 strain of SARS-CoV-2, Cell, doi:10.1016/j.cell.2021.03.055
Imai, Iwatsuki-Horimoto, Hatta, Loeber, Halfmann et al., Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2009799117
Kaku, Narayan, Schmidt, Engler, Li et al., ADG20, a half-life-extended monoclonal antibody in development for the prevention and treatment of COVID-19, demonstrated broad in vitro neutralisation against SARS-CoV-2 variants
Liu, Ginn, Dejnirattisai, Supasa, Wang et al., Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum, Cell, doi:10.1016/j.cell.2021.06.020
Liu, Iketani, Guo, Chan, Wang et al., Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2, Nature, doi:10.1038/s41586-021-04388-0
Martinez, Schäfer, Gobeil, Li, De La Cruz et al., A broadly cross-reactive antibody neutralizes and protects against sarbecovirus challenge in mice, Sci Transl Med, doi:10.1126/scitranslmed.abj7125
Osterrieder, Bertzbach, Dietert, Abdelgawad, Vladimirova et al., Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters, Viruses, doi:10.3390/v12070779
Rappazzo, Tse, Kaku, Wrapp, Sakharkar et al., Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody, Science, doi:10.1126/science.abf4830
Rogers, Zhao, Huang, Beutler, Burns et al., Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model, Science, doi:10.1126/science.abc7520
Sia, Yan, Chin, Fung, Choy et al., Pathogenesis and transmission of SARS-CoV-2 in golden hamsters, Nature, doi:10.1038/s41586-020-2342-5
Vegivinti, Evanson, Lyons, Akosman, Barrett et al., Efficacy of antiviral therapies for COVID-19: a systematic review of randomized controlled trials, BMC Infect Dis, doi:10.1186/s12879-022-07068-0
Who, Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update, WHO
DOI record:
{
"DOI": "10.1128/aac.01353-22",
"ISSN": [
"0066-4804",
"1098-6596"
],
"URL": "http://dx.doi.org/10.1128/aac.01353-22",
"abstract": "<jats:p>Adintrevimab is a human immunoglobulin G1 monoclonal antibody engineered to have broad neutralization against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and other SARS-like coronaviruses with pandemic potential. In both Syrian golden hamster and rhesus macaque models, prophylactic administration of a single dose of adintrevimab provided protection against SARS-CoV-2/WA1/2020 infection in a dose-dependent manner, as measured by significant reductions in lung viral load and virus-induced lung pathology, and by inhibition of viral replication in the upper and lower respiratory tract.</jats:p>",
"alternative-id": [
"10.1128/aac.01353-22"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-10-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-11-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-12-15"
}
],
"author": [
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Zumbrun",
"given": "Elizabeth E.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Adimab LLC, Lebanon, New Hampshire, USA"
}
],
"family": "Kaku",
"given": "Chengzi I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc., Waltham, Massachusetts, USA"
}
],
"family": "Dillinger",
"given": "Lukas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Zak",
"given": "Samantha E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Kuehne",
"given": "Ana I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Bakken",
"given": "Russel R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Koehler",
"given": "Jeffrey W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Delp",
"given": "Korey L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Stefan",
"given": "Christopher P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Kumar",
"given": "Raina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Kugelman",
"given": "Jeffrey R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Moreau",
"given": "Alicia M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Zeng",
"given": "Xiankun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Dye",
"given": "John M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "U.S. Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA"
}
],
"family": "Herbert",
"given": "Andrew S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7977-4783",
"affiliation": [
{
"name": "Invivyd, Inc., Waltham, Massachusetts, USA"
}
],
"authenticated-orcid": true,
"family": "Narayan",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc., Waltham, Massachusetts, USA"
}
],
"family": "Walker",
"given": "Laura M.",
"sequence": "additional"
}
],
"container-title": "Antimicrobial Agents and Chemotherapy",
"container-title-short": "Antimicrob Agents Chemother",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2022,
12,
15
]
],
"date-time": "2022-12-15T14:02:42Z",
"timestamp": 1671112962000
},
"deposited": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T14:05:22Z",
"timestamp": 1674569122000
},
"indexed": {
"date-parts": [
[
2023,
7,
25
]
],
"date-time": "2023-07-25T16:52:51Z",
"timestamp": 1690303971674
},
"is-referenced-by-count": 1,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
1,
24
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023,
1,
24
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://doi.org/10.1128/ASMCopyrightv2",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T00:00:00Z",
"timestamp": 1674518400000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T00:00:00Z",
"timestamp": 1674518400000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.01353-22",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.01353-22",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2023,
1,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
1,
24
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"key": "e_1_3_3_2_2",
"unstructured": "WHO. 2022. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. WHO Geneva Switzerland. Available from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports."
},
{
"DOI": "10.1056/NEJMoa2119451",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_3_2"
},
{
"DOI": "10.1038/s41586-021-04388-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_4_2"
},
{
"DOI": "10.1186/s12879-022-07068-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_5_2"
},
{
"DOI": "10.1016/j.cell.2021.03.055",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_6_2"
},
{
"DOI": "10.1016/j.cell.2021.06.020",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_7_2"
},
{
"DOI": "10.1126/science.abf4830",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_8_2"
},
{
"DOI": "10.1016/j.cell.2021.12.046",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_9_2"
},
{
"author": "Kaku CI",
"key": "e_1_3_3_10_2",
"unstructured": "Kaku CI, Narayan K, Schmidt P, Engler F, Li Y, Walker LM. 2022. ADG20, a half-life-extended monoclonal antibody in development for the prevention and treatment of COVID-19, demonstrated broad in vitro neutralisation against SARS-CoV-2 variants. Abstract. European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Lisbon, Portugal.",
"volume-title": "ADG20, a half-life-extended monoclonal antibody in development for the prevention and treatment of COVID-19, demonstrated broad in vitro neutralisation against SARS-CoV-2 variants.",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abj7125",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_11_2"
},
{
"author": "National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals",
"edition": "8",
"key": "e_1_3_3_12_2",
"unstructured": "National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. Available from https://www.ncbi.nlm.nih.gov/books/NBK54050/.",
"volume-title": "Guide for the care and use of laboratory animals",
"year": "2011"
},
{
"DOI": "10.1073/pnas.2009799117",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_2"
},
{
"DOI": "10.3390/v12070779",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_14_2"
},
{
"DOI": "10.1038/s41586-020-2342-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_2"
},
{
"DOI": "10.1126/science.abc7520",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_16_2"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/aac.01353-22"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Prophylactic Administration of the Monoclonal Antibody Adintrevimab Protects against SARS-CoV-2 in Hamster and Non-Human Primate Models of COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "67"
}