SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies
et al., bioRxiv, doi:10.1101/2022.02.15.480166, Feb 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
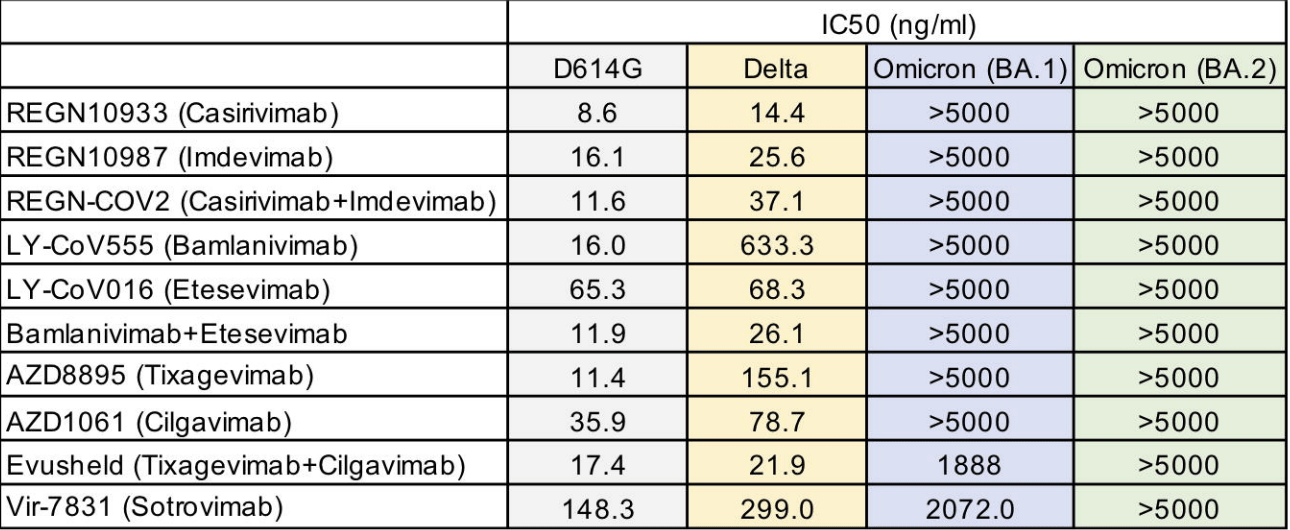
In vitro study showing that omicron BA.2 evades all monoclonal antibodies tested, including sotrovimab and tixagevimab/cilgavimab which retained activity for omicron BA.1.
Study covers sotrovimab, casirivimab/imdevimab, and bamlanivimab/etesevimab.
Zhou et al., 16 Feb 2022, preprint, 4 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Neutralization of SARS-CoV-2 Omicron BA.2 by Therapeutic Monoclonal Antibodies
doi:10.1101/2022.02.15.480166
Monoclonal antibody therapy for the treatment of SARS-CoV-2 infection has been highly successful in decreasing disease severity; however, the recent emergence of the heavily mutated Omicron variant has posed a challenge to this treatment strategy. The Omicron variant BA.1 has been found to evade neutralization by the Regeneron and Eli Lilly therapeutic monoclonal antibodies, while Sotrovimab and the Evusheld monoclonal
References
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Cdc, Omicron Variant: What You Need to Know
Clinicaltrials, Gov, phase III Double-blind, Placebo-controlled Study of AZD7442
Group, Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial, Lancet Infect Dis
Li, Wang, Lavrijsen, Lamers, De Vries et al., SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination, Cell Research
Liu, Iketani, Guo, Chan, Wang et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, Nature
Lyngse, Kirkeby, Denwood, Christiansen, Mølbak et al., Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: Evidence from Danish Households, medRxiv
Majumdar, Sarkar, Mutational and phylogenetic analyses of the two lineages of the Omicron variant, J Med Virol
Noval, Mg, Kaczmarek, Koide, Rodriguez-Rodriguez et al., Antibody isotype diversity against SARS-CoV-2 is associated with differential serum neutralization capacities
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19, Science
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature
Tada, Fan, Chen, Kaur, Stapleford et al., An ACE2
Tada, Zhou, Dcosta, Samanovic, Chivukula et al., Increased resistance of SARS-CoV-2 Omicron Variant to Neutralization by Vaccine-Elicited and Therapeutic Antibodies, bioRxiv
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Res Sq
DOI record:
{
"DOI": "10.1101/2022.02.15.480166",
"URL": "http://dx.doi.org/10.1101/2022.02.15.480166",
"abstract": "<jats:p>Monoclonal antibody therapy for the treatment of SARS-CoV-2 infection has been highly successful in decreasing disease severity; however, the recent emergence of the heavily mutated Omicron variant has posed a challenge to this treatment strategy. The Omicron variant BA.1 has been found to evade neutralization by the Regeneron and Eli Lilly therapeutic monoclonal antibodies, while Sotrovimab and the Evusheld monoclonal antibody cocktail retain significant neutralizing activity. A newly emerged variant, Omicron BA.2, containing the BA.1 mutations plus an additional 6 mutations and 3 deletions, 3 of which lie in the receptor binding domain, has been found to be spreading with increased transmissibility. We report here, using a spike protein-pseudotyped lentivirus assay, that Omicron BA.2 is not neutralized with detectable titer by any of the therapeutic monoclonal antibodies, including Sotrovimab and the Evusheld monoclonal antibodies. The results demonstrate the difficulty of identifying broadly neutralizing monoclonal antibodies against SARS-CoV-2 and the importance of the T cell response from which immunoevasion is more difficult.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
2,
16
]
]
},
"author": [
{
"affiliation": [],
"family": "Zhou",
"given": "Hao",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-0779-9954",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tada",
"given": "Takuya",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8497-4942",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dcosta",
"given": "Belinda M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9997-1004",
"affiliation": [],
"authenticated-orcid": false,
"family": "Landau",
"given": "Nathaniel R",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
16
]
],
"date-time": "2022-02-16T21:50:20Z",
"timestamp": 1645048220000
},
"deposited": {
"date-parts": [
[
2022,
2,
16
]
],
"date-time": "2022-02-16T21:50:20Z",
"timestamp": 1645048220000
},
"group-title": "Immunology",
"indexed": {
"date-parts": [
[
2022,
2,
16
]
],
"date-time": "2022-02-16T22:16:08Z",
"timestamp": 1645049768295
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
2,
16
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.02.15.480166",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
2,
16
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
2,
16
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies"
],
"type": "posted-content"
}
zhou5
