Mortality Predictors Of Pre-variant SARS-CoV-2 Infected ARDS Patients Receiving Favipiravir and Tocilizumab
et al., Research Square, doi:10.21203/rs.3.rs-1666161/v1, May 2022
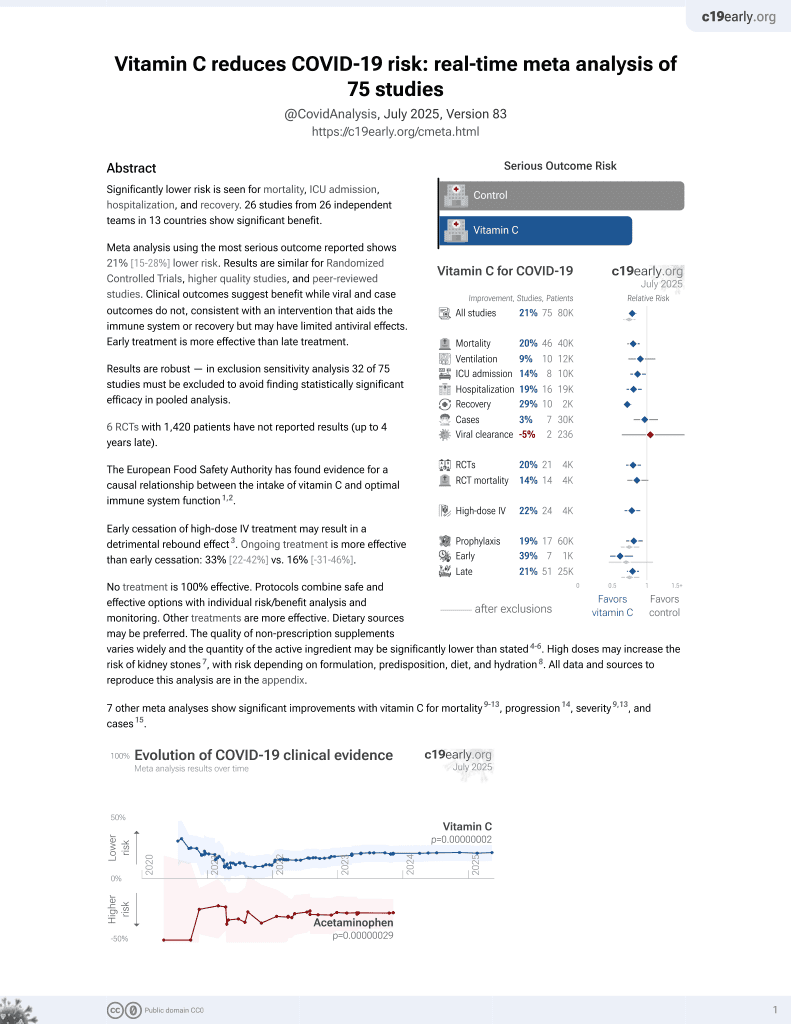
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
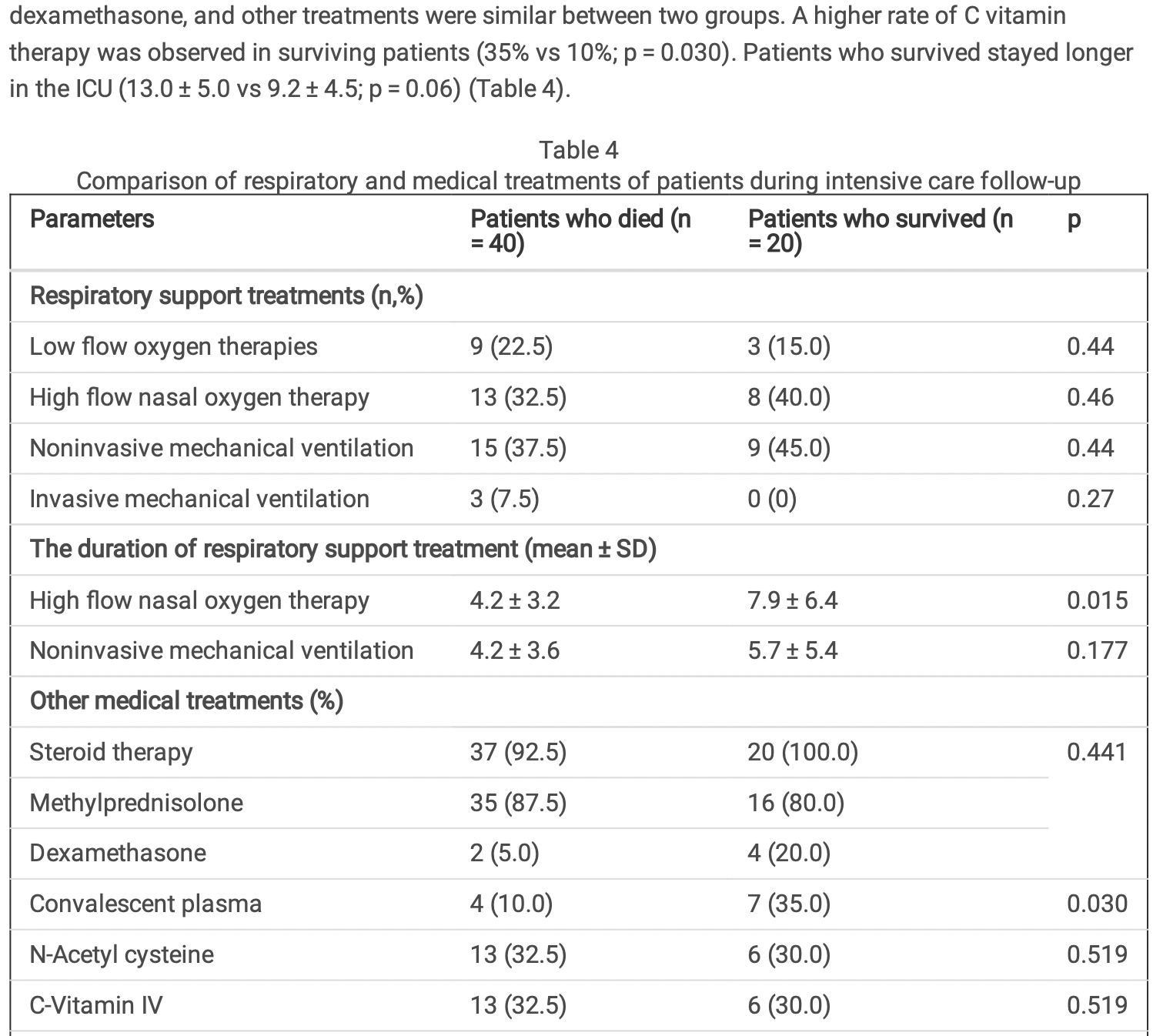
Retrospective 60 ICU patients in Turkey treated with tocilizumab and favipiravir, reporting that there was a higher rate of vitamin C treatment in surviving patients (35% vs 10%; p = 0.03), however the results in the table do not match. The numbers for convalescent plasma and vitamin C may have been switched (the number of patients treated with convalescent plasma also does not match between tables 1 and 4).
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
Yildirim et al., 18 May 2022, retrospective, Turkey, preprint, 5 authors, study period 1 July, 2020 - 5 October, 2020.
Contact: ftagassi@hotmail.com.
Mortality Predictors Of Pre-variant SARS-CoV-2 Infected ARDS Patients Receiving Favipiravir and Tocilizumab
doi:10.21203/rs.3.rs-1666161/v1
In this study, viral clearance (oronasopharyngeal swab RT-PCR negativity) and intensive care outcomes and risk factors affecting mortality of critically ill patients with COVID-19-related acute respiratory distress syndrome (ARDS) who received tocilizumab and favipiravir treatments together before vaccination were investigated.
Material-Methods The data of patients who were followed up and treated between 1 July 2020 and 5 October 2020 were retrospectively analyzed. Demographic data of the patients (age, gender), oro-nasopharyngeal swab RT-PCR and classi cation of ARDS, respiratory support treatments, all medical treatments, and ICU outcomes were recorded.
Results Totally, 60 patients with a median age of 69.8 [24-87], 25 females and 35 males were included in the study. Mean APACHE II score was 18.9 ± 8.0; and SOFA score was 4.5 ± 2.0. Thirty-four (56.7%) patients were intubated during follow-up. Tocilizumab was given on average of 2.5th day (± 2.0 days). On the day of tocilizumab administration, 1 (1.7%) patient had mild ARDS, 30 (50.0%) had moderate ARDS, 29 (48.3%) had severe ARDS. PaO2/FIO2 on the day of tocilizumab administration was 96.7 ± 36.6 mmHg. Forty (66.7%) patients died, while 20 (33.3%) patients transferred to the service. The mean length of stay in the ICU was 11.4 ± 5.5 days. Advanced age [Hazard ratio (HR) 1.8; 95% con dense interval (CI) 0.88-0.93; p < 0.001), higher APACHE II score (HR 0.81, 95% CI 0.74-0.98; p = 0.001), higher SOFA score on the day of tocilizumab administration (HR 1.47, 95% CI 0.39-0.79; p = 0.001), and lower PaO2/FIO2 ratio (HR 2.54, 95% CI 2.33-3.79; p < 0.001) were determined as independent risk factors for mortality.
Conclusion Patients who were administered tocilizumab and favipiravir together in our intensive care unit were mostly patients with severe ARDS and had higher in ammatory markers. High mortality was attributed to the use of tocilizumab as an add-on treatment, not as a routine treatment.
Declarations Con ict of interest The authors declare that there is no con ict of interest regarding the publication of this paper.
Ethics Statement This study was performed in accordance with the ethical principles in the Good Clinical Practice (GCP) guidelines and Declaration of Helsinki, applicable local regulatory requirements and the protocol were approved by Ethics Committee of tertiary institution.
Authors' contributions All authors have contributed su ciently in the conception and design of the study, data collection, and interpretation as well as the preparation of the manuscript. All authors read and approved the nal version of the manuscript.
References
Alattar, Ibrahim, Shaar, Abdalla, Shukri et al., Tocilizumab for the treatment of severe coronavirus disease 2019, J Med Virol
Bhatraju, Ghassemieh, Nichols, Kim, Jerome et al., Covid-19 in Critically Ill Patients in the Seattle Region -Case Series, N Engl J Med
Buonaguro, Puzanov, Ascierto, Anti-IL6R role in treatment of COVID-19-related ARDS, Journal of Translational Medicine
Cascella, Mauro, Blasio, Crispo, Gaudio et al., Rapid and Impressive Response to a Combined Treatment with Single-Dose Tocilizumab and NIV in a Patient with COVID-19 Pneumonia/ARDS, Medicina (Kaunas)
Gordon, Mouncey, Al-Beidh, Rowan, Nichol et al., Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19, N Engl J Med
Hermine, Tharaux, Resche-Rigon, Porcher, Ravaud, Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial, JAMA Intern Med
Hsu, Mao, Liu, Lai, Pharmacology and Adverse Events of Emergency-Use Authorized Medication in Moderate to Severe COVID-19, Pharmaceuticals
Jordan, Zakowski, Tran, Smith, Gaultier et al., Compassionate Use of Tocilizumab for Treatment of SARS-CoV-2 Pneumonia, Clin Infect Dis
Koutsakos, Kedzierska, A race to determine what drives COVID-19 severity, Nature
Netea, Rovina, Akinosoglou, Antoniadou, Antonakos, Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure, Cell Host Microbe
Sciascia, Aprà, Baffa, Baldovino, Boaro et al., Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19, Clin Exp Rheumatol
Somers, Eschenauer, Troost, Golob, Gandhi et al., Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19, Clin Infect Dis
Stone, Frigault, Serling-Boyd, Fernandes, Harvey et al., E cacy of Tocilizumab in Patients Hospitalized with Covid-19, N Engl J Med
Sun, Wang, Cai, Hu, Chen et al., Cytokine storm intervention in the early stages of COVID-19 pneumonia, Cytokine Growth Factor Rev
Toniati, Piva, Cattalini, Garrafa, Regola et al., Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperin ammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy, Autoimmun Rev
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, Jama
Yang, Ding, Xu, Pu, Li et al., Increased circulating level of interleukin-6 and CD8(+) T cell exhaustion are associated with progression of COVID-19, Infect Dis Poverty
Yang, Yu, Xu, Shu, Xia et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. which further evaluates e cacy of tocilizumab in critical COVID-19 ARDS patients, Lancet Respir Med
Zhang, Song, Tong, Fei, Guo et al., First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab, Blood Adv
Zhao, Cui, Tian, E cacy of tocilizumab treatment in severely ill COVID-19 patients, Crit Care
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.21203/rs.3.rs-1666161/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-1666161/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Objective\n In this study, viral clearance (oronasopharyngeal swab RT-PCR negativity) and intensive care outcomes and risk factors affecting mortality of critically ill patients with COVID-19-related acute respiratory distress syndrome (ARDS) who received tocilizumab and favipiravir treatments together before vaccination were investigated.\nMaterial-Methods\n The data of patients who were followed up and treated between 1 July 2020 and 5 October 2020 were retrospectively analyzed. Demographic data of the patients (age, gender), oro-nasopharyngeal swab RT-PCR and classification of ARDS, respiratory support treatments, all medical treatments, and ICU outcomes were recorded.\nResults\n Totally, 60 patients with a median age of 69.8 [24–87], 25 females and 35 males were included in the study. Mean APACHE II score was 18.9 ± 8.0; and SOFA score was 4.5 ± 2.0. Thirty-four (56.7%) patients were intubated during follow-up. Tocilizumab was given on average of 2.5th day (± 2.0 days). On the day of tocilizumab administration, 1 (1.7%) patient had mild ARDS, 30 (50.0%) had moderate ARDS, 29 (48.3%) had severe ARDS. PaO2/FIO2 on the day of tocilizumab administration was 96.7 ± 36.6 mmHg. Forty (66.7%) patients died, while 20 (33.3%) patients transferred to the service. The mean length of stay in the ICU was 11.4 ± 5.5 days. Advanced age [Hazard ratio (HR) 1.8; 95% confidense interval (CI) 0.88–0.93; p < 0.001), higher APACHE II score (HR 0.81, 95% CI 0.74–0.98; p = 0.001), higher SOFA score on the day of tocilizumab administration (HR 1.47, 95% CI 0.39–0.79; p = 0.001), and lower PaO2/FIO2 ratio (HR 2.54, 95% CI 2.33–3.79; p < 0.001) were determined as independent risk factors for mortality.\nConclusion\n Patients who were administered tocilizumab and favipiravir together in our intensive care unit were mostly patients with severe ARDS and had higher inflammatory markers. High mortality was attributed to the use of tocilizumab as an add-on treatment, not as a routine treatment.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
5,
17
]
]
},
"author": [
{
"affiliation": [
{
"name": "University of Health Sciences Dışkapı Yıldırım Beyazıt Training and Research Hospital, Department of Chest Diseases, COVID Intensive Care Unit, Ankara, Turkey"
}
],
"family": "YILDIRIM",
"given": "Fatma",
"sequence": "first"
},
{
"affiliation": [
{
"name": "University of Health Sciences Dışkapı Yıldırım Beyazıt Training and Research Hospital, Department of Internal Medicine, COVID Intensive Care Unit, Ankara, Turkey"
}
],
"family": "ŞİMŞEK",
"given": "Meltem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Health Sciences Dışkapı Yıldırım Beyazıt Training and Research Hospital, Department of General Surgery, COVID Intensive Care Unit, Ankara, Turkey"
}
],
"family": "APAYDIN",
"given": "Muhammed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bahcesehir University School of Medicine, Istanbul, Turkey"
}
],
"family": "KARAMAN",
"given": "İrem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Health Sciences Dışkapı Yıldırım Beyazıt Training and Research Hospital, Department of General Surgery, COVID Intensive Care Unit, Ankara, Turkey"
}
],
"family": "DURAL",
"given": "Halil İbrahim",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T15:31:01Z",
"timestamp": 1652887861000
},
"deposited": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T15:31:03Z",
"timestamp": 1652887863000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T16:12:15Z",
"timestamp": 1652890335405
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5,
18
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
18
]
],
"date-time": "2022-05-18T00:00:00Z",
"timestamp": 1652832000000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-1666161/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-1666161/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
5,
18
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2022,
5,
18
]
]
},
"publisher": "Research Square Platform LLC",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-1666161/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Mortality Predictors Of Pre-variant SARS-CoV-2 Infected ARDS Patients Receiving Favipiravir and Tocilizumab",
"type": "posted-content"
}