A study of impurities in the repurposed COVID-19 drug hydroxychloroquine sulfate by UHPLC-Q/TOF-MS and LC-SPE-NMR
et al., Rapid Communications in Mass Spectrometry, doi:10.1002/rcm.9358, Jul 2022
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
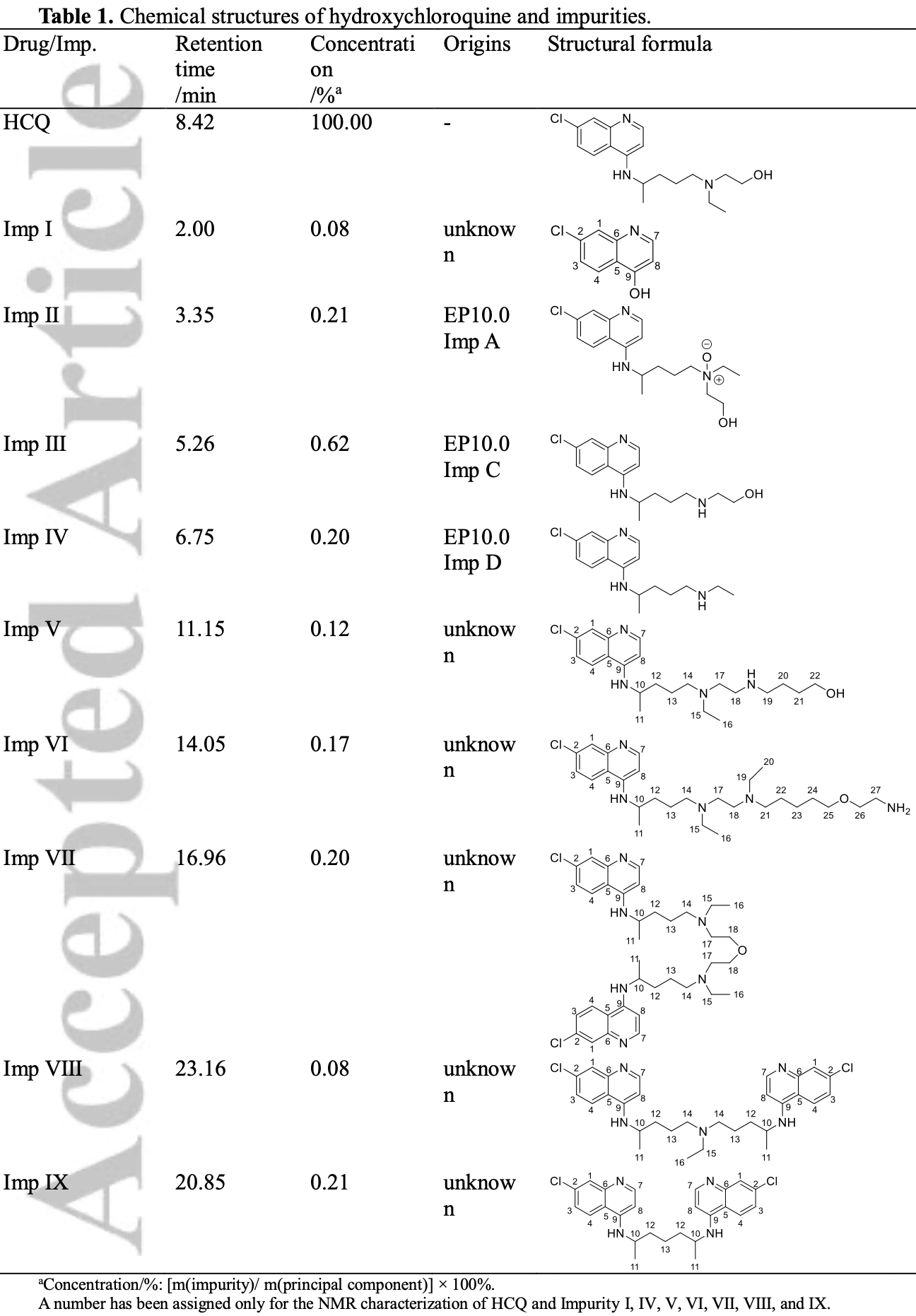
Analysis of HCQ from two manufacturers showing 9 different impurities, with significantly different concentrations for each manufacturer.
Xu et al., 26 Jul 2022, peer-reviewed, 4 authors.
Contact: clare_ruan@163.com, sunnan@zjut.edu.cn.
A study of impurities in the repurposed COVID‐19 drug hydroxychloroquine sulfate using ultra‐high‐performance liquid chromatography‐quadrupole/time‐of‐flight mass spectrometry and liquid chromatography‐solid‐phase extraction‐nuclear magnetic resonance
Rapid Communications in Mass Spectrometry, doi:10.1002/rcm.9358
Rationale: Hydroxychloroquine sulfate is effective in the treatment of malaria, autoimmune diseases, and as an antiviral drug. However, unreported impurities are often detected in this drug, which pose a health risk. In this study, the structures of hydroxychloroquine and six unknown impurities were analyzed using ultra-high performance liquid chromatographyquadrupole/time-of-flight tandem mass spectrometry (UHPLC-Q/TOF MS), and the structures were characterized using liquid chromatography-solid-phase extraction-nuclear magnetic resonance spectroscopy (LC-SPE-NMR).
Methods: The column was an Agilent InfinityLad Poroshell HPH-C18 (100 mm × 4.6 mm, 2.7 µm). For the analysis of hydroxychloroquine and six unknown impurities, the mobile phase was 20 mM ammonium formate aqueous solution and methanol/acetonitrile (80:20, v/v), using gradient elution. Full-scan MS and MS 2 were performed in order to obtain as much structural information as possible. Additionally, six unknown impurities were separated by semi-preparative liquid chromatography and characterized by LC-SPE-NMR.
Results: The MS 2 fragmentation patterns of the impurities were investigated, leading to more structural information and an understanding of the fragmentation pathways of the impurities. The unknown impurities' structures were confirmed by NMR. In addition, some possible This article is protected by copyright. All rights reserved. pathways of the formation of the impurities in the drugs were outlined, and these impurities were found to be process impurities.
Conclusions: Based on the identification and characterization of these impurities, this study also describes the cause of the production of the impurities and provides insights for companies to improve their production processes and a scientific basis for the improvement of the related pharmacopoeias.
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Accinelli, Ynga-Meléndez, León-Abarca, Hydroxychloroquine/ azithromycin in COVID-19: The association between time to treatment and case fatality rate, Trav. Med. and Infect. Dis, doi:10.1016/j.tmaid.2021.102163
Bodur, Erarpat, Günkara, Bakırdere, Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine, serum and saliva samples by GC-MS, J. Pharm. Anal, doi:10.1016/j.jpha.2021.01.006
Bodur, Erarpat, Günkara, Bakırdere, One step derivatization and dispersive liquid-liquid microextraction of hydroxychloroquine sulfate for its sensitive and accurate determination using GC-MS, J. Pharmacol. Tox. Met, doi:10.1016/j.vascn.2021.107130
Chang, Piette, Foering, Tenhave, Okawa et al., Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis, Arch. Dermatol, doi:10.1001/archdermatol.2011.19.
Chhonker, Sleightholm, Li, Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC-ESI-MS/MS: An application for pharmacokinetic studies, J. Chromatogr. B, doi:10.1016/j.jchromb.2017.11.026
Christian, Jean-Marc, New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, Int. J. Antimicrob. Ag, doi:10.1016/j.ijantimicag.2020.105938
Dongre, Ghugare, Karmuse, Identification and characterization of process related impurities in chloroquine and hydroxychloroquine by LC/IT/MS, LC/TOF/MS and NMR, J. Pharm. Biomed. Anal, doi:10.1016/j.jpba.2009.01.013
Jacobs, Stammers, Louis, Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in COVID-19: Experience with Patients, ASAIO J, doi:10.1097/MAT.0000000000001185
Kenny, Smyth, Hewage, 4-Hydroxyphenylacetic acid derivatives of inositol from dandelion (Taraxacum officinale) root characterised using LC-SPE-NMR and LC-MS techniques, Phytochemistry, doi:10.1016/j.phytochem.2013.11.022
Mcchesney, Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate, Am. J. Med, doi:10.1016/0002-9343(83)91265-2
Mckinnon, Wang, Zervos, Safety and Tolerability of Hydroxychloroquine in healthcare workers and first responders for the prevention of COVID-19: WHIP COVID-19 Study, Int. J. of Infect. Dis, doi:10.1016/j.ijid.2021.12.343
Narayanam, Sahu, Singh, Use of LC-MS/TOF, LC-MS n , NMR and LC-NMR in characterization of stress degradation products: Application to cilazapril, J. Pharm. Biomed. Anal, doi:10.1016/j.jpba.2015.03.038
Noureddine, Issaoui, Medimagh, Quantum chemical studies on molecular structure, AIM, ELF, RDG and antiviral activities of hybrid hydroxychloroquine in the treatment of COVID-19: Molecular docking and DFT calculations, J. King Saud Univer. -Sci, doi:10.1016/j.jksus.2020.101334
Pendela, Béni, Haghedooren, Combined use of liquid chromatography with mass spectrometry and nuclear magnetic resonance for the identification of degradation compounds in an erythromycin formulation, Anal. Bioanal. Chem, doi:10.1007/s00216-011-5450-0
Recovery, Effect of hydroxychloroquine in hospitalized patients with Covid-19, New England J. of Med, doi:10.1056/NEJMoa2022926
Saini, Bansal, Characterization of four new photodegradation products of hydroxychloroquine through LC-PDA, ESI-MS n and LC-MS-TOF studies, J. Pharm. Biomed. Anal, doi:10.1016/j.jpba.2013.06.014
Seet, Quek, Ooi, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial, Int. J. of Infect. Dis, doi:10.1016/j.ijid.2021.04.035
Sogut, Can, Guven, Safety and efficacy of hydroxychloroquine in 152 outpatients with confirmed COVID-19: A pilot observational study, Am. J. Emerg. Med, doi:10.1016/j.ajem.2020.12.014
Soichot, Mégarbane, Houzé, Development, validation and clinical application of a LC-MS/MS method for the simultaneous quantification of hydroxychloroquine and its active metabolites in human whole blood, J. Pharm. Biomed. Anal, doi:10.1016/j.jpba.2014.07.009
Wahie, Daly, Cordell, Clinical and pharmacogenetic influences on response to hydroxychloroquine in discoid lupus erythematosus: a retrospective cohort study, J. Invest. Dermatol, doi:10.1038/jid.2011.167
Wang, Ong, Chin, Method development and validation for rapid quantification of hydroxychloroquine in human blood using liquid chromatographytandem mass spectrometry, J. Pharm. and Biomed. Anal, doi:10.1016/j.jpba.2011.11.034
Wright, Ross, Goldrick, Are hydroxychloroquine and chloroquine effective in the treatment of SARS-COV-2 (COVID-19) ?, Evid. Based. Dent, doi:10.1093/ofid/ofaa130
Yao, Ye, Zhang, Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis, doi:10.1093/cid/ciaa237
Zeidi, Kim, Werth, Increased Myeloid Dendritic Cells and TNF-a Expression Predicts Poor Response to Hydroxychloroquine in Cutaneous Lupus Erythematosus, J. Invest. Dermatol, doi:10.1016/j.jid.2018.07.041
DOI record:
{
"DOI": "10.1002/rcm.9358",
"ISSN": [
"0951-4198",
"1097-0231"
],
"URL": "http://dx.doi.org/10.1002/rcm.9358",
"alternative-id": [
"10.1002/rcm.9358"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-04-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-07-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-07-26"
}
],
"author": [
{
"affiliation": [
{
"name": "Zhejiang University of Technology Hangzhou China"
}
],
"family": "Xu",
"given": "Donghai",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Zhejiang Institute for Food and Drug Control, National Medical Product Administration Key Laboratory for Core Technology of Generic Drug Evaluation Hangzhou China"
}
],
"family": "Pan",
"given": "Fangfang",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7096-0635",
"affiliation": [
{
"name": "Zhejiang University of Technology Hangzhou China"
},
{
"name": "Zhejiang Institute for Food and Drug Control, National Medical Product Administration Key Laboratory for Core Technology of Generic Drug Evaluation Hangzhou China"
}
],
"authenticated-orcid": false,
"family": "Ruan",
"given": "Hao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Zhejiang University of Technology Hangzhou China"
}
],
"family": "Sun",
"given": "Nan",
"sequence": "additional"
}
],
"container-title": "Rapid Communications in Mass Spectrometry",
"container-title-short": "Rapid Comm Mass Spectrometry",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
26
]
],
"date-time": "2022-07-26T20:29:33Z",
"timestamp": 1658867373000
},
"deposited": {
"date-parts": [
[
2022,
7,
26
]
],
"date-time": "2022-07-26T20:29:33Z",
"timestamp": 1658867373000
},
"indexed": {
"date-parts": [
[
2022,
7,
27
]
],
"date-time": "2022-07-27T04:49:24Z",
"timestamp": 1658897364796
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
26
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
26
]
],
"date-time": "2022-07-26T00:00:00Z",
"timestamp": 1658793600000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
26
]
],
"date-time": "2022-07-26T00:00:00Z",
"timestamp": 1658793600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/rcm.9358",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/rcm.9358",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
7,
26
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
26
]
]
},
"publisher": "Wiley",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/rcm.9358"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Organic Chemistry",
"Spectroscopy",
"Analytical Chemistry"
],
"subtitle": [],
"title": "A study of impurities in the repurposed COVID‐19 drug hydroxychloroquine sulfate by UHPLC‐Q/TOF‐MS and LC‐SPE‐NMR",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
