Impact of Paxlovid on in-hospital outcomes and post-COVID-19 condition in adult patients infected with SARS-CoV-2 Omicron variant: A non-randomized controlled clinical trial
et al., Medicine, doi:10.1097/MD.0000000000036714, ChiCTR2300071537, Dec 2023
Prospective study of 320 COVID-19 patients infected with the SARS-CoV-2 Omicron variant in China, showing improved viral clearance and symptom resolution with 5 days of paxlovid treatment. Authors perform multivariable analysis for post-covid condition but not for the main outcomes.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
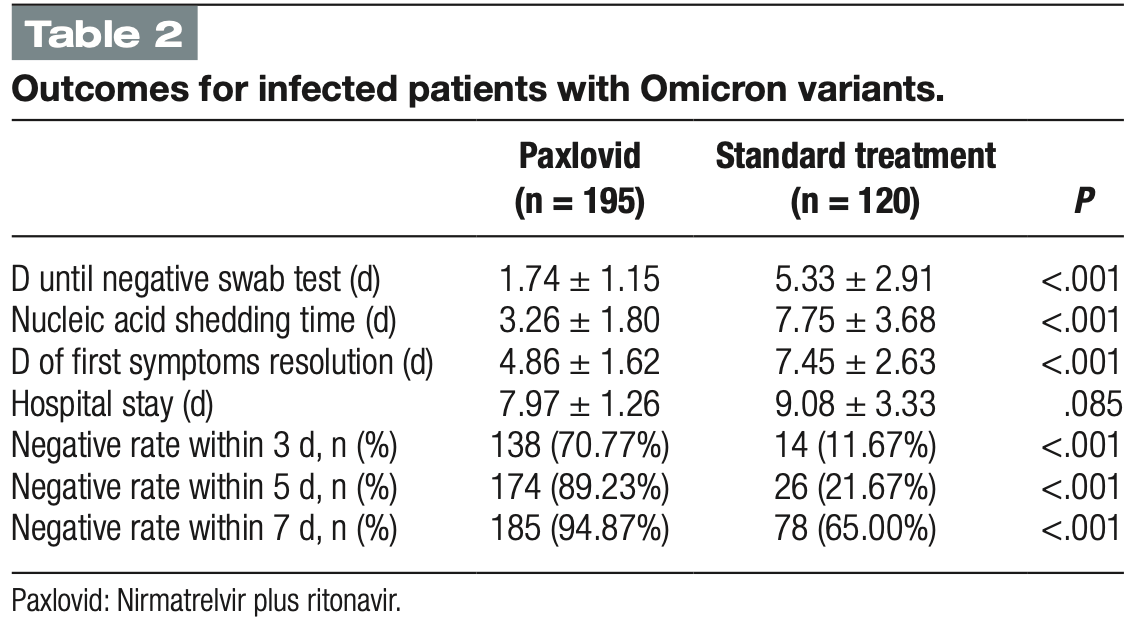
hospitalization time, 12.2% lower, relative time 0.88, p = 0.09, treatment mean 7.97 (±1.26) n=195, control mean 9.08 (±3.33) n=120.
|
|
recovery time, 34.8% lower, relative time 0.65, p < 0.001, treatment mean 4.86 (±1.62) n=195, control mean 7.45 (±2.63) n=120.
|
|
time to viral-, 57.9% lower, relative time 0.42, p < 0.001, treatment mean 3.26 (±1.8) n=195, control mean 7.75 (±3.68) n=120, primary outcome.
|
|
risk of long COVID, 46.9% lower, OR 0.53, p = 0.04, treatment 195, control 120, adjusted per study, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Xu et al., 22 Dec 2023, prospective, China, peer-reviewed, mean age 29.1, 9 authors, study period 5 November, 2022 - 28 November, 2022, trial ChiCTR2300071537.
Contact: drzhyubin@163.com.
Impact of Paxlovid on in-hospital outcomes and post-COVID-19 condition in adult patients infected with SARS-CoV-2 Omicron variant: A non-randomized controlled clinical trial
Medicine, doi:10.1097/md.0000000000036714
Background: Nirmatrelvir plus ritonavir (Paxlovid) have been used in the treatment of adult patients with mild-to-moderate coronavirus disease 2019 (COVID-19). This study aimed to evaluate the impact of Paxlovid on in-hospital outcomes and post-COVID-19 condition in Chinese adult patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant. Methods: This non-randomized clinical controlled trial recruited patients infected with SARS-CoV-2 Omicron variant from the designated hospital for treating COVID-19 between November 5 and November 28, 2022, in Shijiazhuang, China. Participants were administered Paxlovid (300 mg of nirmatrelvir and 100 mg of ritonavir orally) or standard treatment. The primary outcome was the nucleic acid shedding time and post-COVID-19 condition. Results: A total of 320 patients infected with SARS-CoV-2 Omicron variant were included, with mean age of 29.10 ± 7.34 years old. Two hundred patients received Paxlovid. Compared to patients in the standard treatment group, those in Paxlovid group had a significantly shorter nucleic acid shedding time (3.26 ± 1.80 vs 7.75 ± 3.68 days, P < .001), shorter days until negative swab test (1.74 ± 1.15 vs 5.33 ± 2.91, P < .001), shorter days of first symptoms resolution (4.86 ± 1.62 vs 7.45 ± 2.63, P < .001), higher in nucleic acid test negative rate within 3 days [138 (70.77%) vs 14 (11.67%), P < .001], higher negative rate within 5 days [174 (89.23%) vs 26 (21.67%), P < .001], negative rate within 7 days [185 (94.87%) vs 78 (65.00%), P < .001], and were less likely to have post-COVID-19 condition [32 (18.60%) vs 30 (31.57%), P = .016]. There was no significant difference in duration of post-COVID-19 condition (43.00 ± 26.00 vs 49.00 ± 26.34 days, P = .354) between the 2 groups. Conclusion: Compared to standard treatment, Paxlovid significantly reduced nucleic acid shedding time, days until negative swab test, and days of first symptoms resolution, as well as improved nucleic acid test negative rate and post-COVID-19 condition.
References
Ai, Zhang, Zhang, Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost, Emerg Microbes Infect
Azizogli, Pai, Coppola, Scalable inhibitors of the Nsp3-Nsp4 coupling in SARS-CoV-2, ACS Omega
Cao, Gao, Bao, VV116 versus nirmatrelvir-ritonavir for oral treatment of Covid-19, N Engl J Med
Cegolon, Magnano, Negro, SARS-CoV-2 reinfections in health-care workers, 1 March 2020-31, Viruses
Cegolon, Mastrangelo, Bellizzi, Supporting the aspecific physiological defenses of upper airways against emerging SARS-CoV-2 variants, Pathogens
Cegolon, Mastrangelo, Emanuelli, Early negativization of SARS-CoV-2 infection by nasal spray of seawater plus additives: the RENAISSANCE open-label controlled clinical trial, Pharmaceutics
Cegolon, Pol, Simonetti, Molnupiravir, nirmatrelvir/ritonavir, or sotrovimab for high-risk COVID-19 patients infected by the omicron variant: hospitalization, mortality, and time until negative swab test in real life, Pharmaceuticals
Charness, Gupta, Stack, Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment, N Engl J Med
Desgranges, Tadini, Munting, Post-COVID-19 syndrome in outpatients: a cohort study, J Gen Intern Med
Fan, Li, Zhang, SARS-CoV-2 Omicron variant: recent progress and future perspectives, Signal Transduct Target Ther
Fiolet, Kherabi, Macdonald, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review, Clin Microbiol Infect
Goenka, Liu, Infectious diseases, human capital and economic growth, Econ Theory
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl Med
Huang, Li, Gu, Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study, Lancet Respir Med
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jackson, Farzan, Chen, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol
Kandeel, Mohamed, El-Lateef, Omicron variant genome evolution and phylogenetics, J Med Virol
Lai, Hsu, Yen, Long COVID: an inevitable sequela of SARS-CoV-2 infection, J Microbiol Immunol Infect
Lauring, Tenforde, Chappell, Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study, BMJ
Malden, Hong, Lewin, Hospitalization and emergency department encounters for COVID-19 after Paxlovid treatment -California, December 2021-May 2022, MMWR Morb Mortal Wkly Rep
Marzolini, Kuritzkes, Marra, Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications, Clin Pharmacol Ther
Meo, Alhowikan, Al-Khlaiwi, Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV, Eur Rev Med Pharmacol Sci
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis
Oronsky, Larson, Hammond, A review of persistent post-COVID syndrome (PPCS), Clin Rev Allergy Immunol
Seeßle, Waterboer, Hippchen, Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort Study, Clin Infect Dis
Soriano, Murthy, Marshall, A clinical case definition of post-COVID-19 condition by a Delphi consensus, Lancet Infect Dis
Wen, Chen, Tang, Efficacy and safety of three new oral antiviral treatment (Molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis, Ann Med
Xu, None, Medicine
Zhao, Lu, Peng, SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells, Emerg Microbes Infect
Zheng, Ma, Wang, Efficacy and safety of Paxlovid for COVID-19:a meta-analysis, J Infect
Zhou, Zhi, Teng, The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity, J Med Virol
Ziegler, Allon, Nyquist, SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues, Cell
DOI record:
{
"DOI": "10.1097/md.0000000000036714",
"ISSN": [
"0025-7974",
"1536-5964"
],
"URL": "http://dx.doi.org/10.1097/MD.0000000000036714",
"abstract": "<jats:sec>\n <jats:title>Background:</jats:title>\n <jats:p>Nirmatrelvir plus ritonavir (Paxlovid) have been used in the treatment of adult patients with mild-to-moderate coronavirus disease 2019 (COVID-19). This study aimed to evaluate the impact of Paxlovid on in-hospital outcomes and post-COVID-19 condition in Chinese adult patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods:</jats:title>\n <jats:p>This non-randomized clinical controlled trial recruited patients infected with SARS-CoV-2 Omicron variant from the designated hospital for treating COVID-19 between November 5 and November 28, 2022, in Shijiazhuang, China. Participants were administered Paxlovid (300 mg of nirmatrelvir and 100 mg of ritonavir orally) or standard treatment. The primary outcome was the nucleic acid shedding time and post-COVID-19 condition.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results:</jats:title>\n <jats:p>A total of 320 patients infected with SARS-CoV-2 Omicron variant were included, with mean age of 29.10 ± 7.34 years old. Two hundred patients received Paxlovid. Compared to patients in the standard treatment group, those in Paxlovid group had a significantly shorter nucleic acid shedding time (3.26 ± 1.80 vs 7.75 ± 3.68 days, <jats:italic toggle=\"yes\">P</jats:italic> < .001), shorter days until negative swab test (1.74 ± 1.15 vs 5.33 ± 2.91, <jats:italic toggle=\"yes\">P</jats:italic> < .001), shorter days of first symptoms resolution (4.86 ± 1.62 vs 7.45 ± 2.63, <jats:italic toggle=\"yes\">P</jats:italic> < .001), higher in nucleic acid test negative rate within 3 days [138 (70.77%) vs 14 (11.67%), <jats:italic toggle=\"yes\">P</jats:italic> < .001], higher negative rate within 5 days [174 (89.23%) vs 26 (21.67%), <jats:italic toggle=\"yes\">P</jats:italic> < .001], negative rate within 7 days [185 (94.87%) vs 78 (65.00%), <jats:italic toggle=\"yes\">P</jats:italic> < .001], and were less likely to have post-COVID-19 condition [32 (18.60%) vs 30 (31.57%), <jats:italic toggle=\"yes\">P</jats:italic> = .016]. There was no significant difference in duration of post-COVID-19 condition (43.00 ± 26.00 vs 49.00 ± 26.34 days, <jats:italic toggle=\"yes\">P</jats:italic> = .354) between the 2 groups.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion:</jats:title>\n <jats:p>Compared to standard treatment, Paxlovid significantly reduced nucleic acid shedding time, days until negative swab test, and days of first symptoms resolution, as well as improved nucleic acid test negative rate and post-COVID-19 condition.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Hebei University of Chinese Medicine, Shijiazhuang, China"
},
{
"name": "Shijiazhuang People’s Hospital, Shijiazhuang, China"
}
],
"family": "Xu",
"given": "Jianchao",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Hebei University of Chinese Medicine, Shijiazhuang, China"
},
{
"name": "The Traditional Chinese Medicine Hospital of Shijiazhuang, Shijiazhuang, China"
}
],
"family": "Song",
"given": "Jinzhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hebei Medical University, Shijiazhuang, China"
}
],
"family": "Xie",
"given": "Ziyu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hebei General Hospital, Shijiazhuang, China"
}
],
"family": "Yang",
"given": "Jie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Traditional Chinese Medicine Hospital of Shijiazhuang, Shijiazhuang, China"
}
],
"family": "Wu",
"given": "Di",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hebei Academy of Chinese Medical Sciences, Shijiazhuang, China"
}
],
"family": "Liu",
"given": "Fengshuang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK"
}
],
"family": "Zhao",
"given": "Yinuo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Traditional Chinese Medicine Hospital of Shijiazhuang, Shijiazhuang, China"
}
],
"family": "Zang",
"given": "Hongmin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1981-9297",
"affiliation": [
{
"name": "Hebei University of Chinese Medicine, Shijiazhuang, China"
},
{
"name": "Shijiazhuang People’s Hospital, Shijiazhuang, China"
},
{
"name": "North China University of Science and Technology, Tangshan, China."
}
],
"authenticated-orcid": false,
"family": "Zhao",
"given": "Yubin",
"sequence": "additional"
}
],
"container-title": "Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T19:01:23Z",
"timestamp": 1703271683000
},
"deposited": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T19:01:37Z",
"timestamp": 1703271697000
},
"indexed": {
"date-parts": [
[
2023,
12,
23
]
],
"date-time": "2023-12-23T00:21:08Z",
"timestamp": 1703290868783
},
"is-referenced-by-count": 0,
"issue": "51",
"issued": {
"date-parts": [
[
2023,
12,
22
]
]
},
"journal-issue": {
"issue": "51",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T00:00:00Z",
"timestamp": 1703203200000
}
}
],
"link": [
{
"URL": "https://journals.lww.com/10.1097/MD.0000000000036714",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "e36714",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2023,
12,
22
]
]
},
"published-print": {
"date-parts": [
[
2023,
12,
22
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"article-title": "Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV.",
"author": "Meo",
"first-page": "2012",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "R1-20231222",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1007/s00199-019-01214-7",
"article-title": "Infectious diseases, human capital and economic growth.",
"author": "Goenka",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Econ Theory",
"key": "R2-20231222",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.1002/jmv.28138",
"article-title": "The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity.",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "e28138",
"journal-title": "J Med Virol",
"key": "R4-20231222",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1038/s41392-022-00997-x",
"article-title": "SARS-CoV-2 Omicron variant: recent progress and future perspectives.",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Signal Transduct Target Ther",
"key": "R5-20231222",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27515",
"article-title": "Omicron variant genome evolution and phylogenetics.",
"author": "Kandeel",
"doi-asserted-by": "crossref",
"first-page": "1627",
"journal-title": "J Med Virol",
"key": "R6-20231222",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.3390/v15071551",
"article-title": "SARS-CoV-2 reinfections in health-care workers, 1 March 2020–31 January 2023.",
"author": "Cegolon",
"doi-asserted-by": "crossref",
"first-page": "1551",
"journal-title": "Viruses",
"key": "R7-20231222",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1080/22221751.2021.2022440",
"article-title": "Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost.",
"author": "Ai",
"doi-asserted-by": "crossref",
"first-page": "337",
"journal-title": "Emerg Microbes Infect",
"key": "R8-20231222",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2021.10.005",
"article-title": "Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review.",
"author": "Fiolet",
"doi-asserted-by": "crossref",
"first-page": "202",
"journal-title": "Clin Microbiol Infect",
"key": "R9-20231222",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.3390/ph16050721",
"article-title": "Molnupiravir, nirmatrelvir/ritonavir, or sotrovimab for high-risk COVID-19 patients infected by the omicron variant: hospitalization, mortality, and time until negative swab test in real life.",
"author": "Cegolon",
"doi-asserted-by": "crossref",
"first-page": "721",
"journal-title": "Pharmaceuticals (Basel)",
"key": "R10-20231222",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19.",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "R11-20231222",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1021/acsomega.2c06384",
"article-title": "Scalable inhibitors of the Nsp3-Nsp4 coupling in SARS-CoV-2.",
"author": "Azizogli",
"doi-asserted-by": "crossref",
"first-page": "5349",
"journal-title": "ACS Omega",
"key": "R12-20231222",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications.",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin Pharmacol Ther",
"key": "R13-20231222",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients.",
"author": "Najjar-Debbiny",
"doi-asserted-by": "crossref",
"first-page": "e342",
"journal-title": "Clin Infect Dis",
"key": "R14-20231222",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"article-title": "Efficacy and safety of three new oral antiviral treatment (Molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis.",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Ann Med",
"key": "R15-20231222",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2208822",
"article-title": "VV116 versus nirmatrelvir-ritonavir for oral treatment of Covid-19.",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "N Engl J Med",
"key": "R16-20231222",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2022.09.027",
"article-title": "Efficacy and safety of Paxlovid for COVID-19:a meta-analysis.",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "J Infect",
"key": "R17-20231222",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(21)00703-9",
"article-title": "A clinical case definition of post-COVID-19 condition by a Delphi consensus.",
"author": "Soriano",
"doi-asserted-by": "crossref",
"first-page": "e102",
"journal-title": "Lancet Infect Dis",
"key": "R18-20231222",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2206449",
"article-title": "Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment.",
"author": "Charness",
"doi-asserted-by": "crossref",
"first-page": "1045",
"journal-title": "N Engl J Med",
"key": "R19-20231222",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.3390/pathogens12020211",
"article-title": "Supporting the aspecific physiological defenses of upper airways against emerging SARS-CoV-2 variants.",
"author": "Cegolon",
"doi-asserted-by": "crossref",
"first-page": "211",
"journal-title": "Pathogens",
"key": "R20-20231222",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3390/pharmaceutics14112502",
"article-title": "Early negativization of SARS-CoV-2 infection by nasal spray of seawater plus additives: the RENAISSANCE open-label controlled clinical trial.",
"author": "Cegolon",
"doi-asserted-by": "crossref",
"first-page": "2502",
"journal-title": "Pharmaceutics",
"key": "R21-20231222",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00126-6",
"article-title": "Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study.",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "863",
"journal-title": "Lancet Respir Med",
"key": "R22-20231222",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciab611",
"article-title": "Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort Study.",
"author": "Seeßle",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin Infect Dis",
"key": "R23-20231222",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2021-069761",
"article-title": "Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study.",
"author": "Lauring",
"doi-asserted-by": "crossref",
"first-page": "e069761",
"journal-title": "BMJ",
"key": "R24-20231222",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1007/s11606-021-07242-1",
"article-title": "Post-COVID-19 syndrome in outpatients: a cohort study.",
"author": "Desgranges",
"doi-asserted-by": "crossref",
"first-page": "1943",
"journal-title": "J Gen Intern Med",
"key": "R25-20231222",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1007/s12016-021-08848-3",
"article-title": "A review of persistent post-COVID syndrome (PPCS).",
"author": "Oronsky",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "Clin Rev Allergy Immunol",
"key": "R26-20231222",
"volume": "64",
"year": "2023"
},
{
"DOI": "10.1016/j.jmii.2022.10.003",
"article-title": "Long COVID: an inevitable sequela of SARS-CoV-2 infection.",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J Microbiol Immunol Infect",
"key": "R27-20231222",
"volume": "56",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "R28-20231222",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2021.2023329",
"article-title": "SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells.",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Emerg Microbes Infect",
"key": "R29-20231222",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells.",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "R30-20231222",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.04.035",
"article-title": "SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues.",
"author": "Ziegler",
"doi-asserted-by": "crossref",
"first-page": "1016",
"journal-title": "Cell",
"key": "R31-20231222",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm7125e2",
"article-title": "Hospitalization and emergency department encounters for COVID-19 after Paxlovid treatment – California, December 2021-May 2022.",
"author": "Malden",
"doi-asserted-by": "crossref",
"first-page": "830",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "R32-20231222",
"volume": "71",
"year": "2022"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/MD.0000000000036714"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Impact of Paxlovid on in-hospital outcomes and post-COVID-19 condition in adult patients infected with SARS-CoV-2 Omicron variant: A non-randomized controlled clinical trial",
"type": "journal-article",
"volume": "102"
}