Glycemic Control and Clinical Outcomes in U.S. Patients With COVID-19: Data From the National COVID Cohort Collaborative (N3C) Database
et al., Diabetes Care, doi:10.2337/dc21-2186, Feb 2022
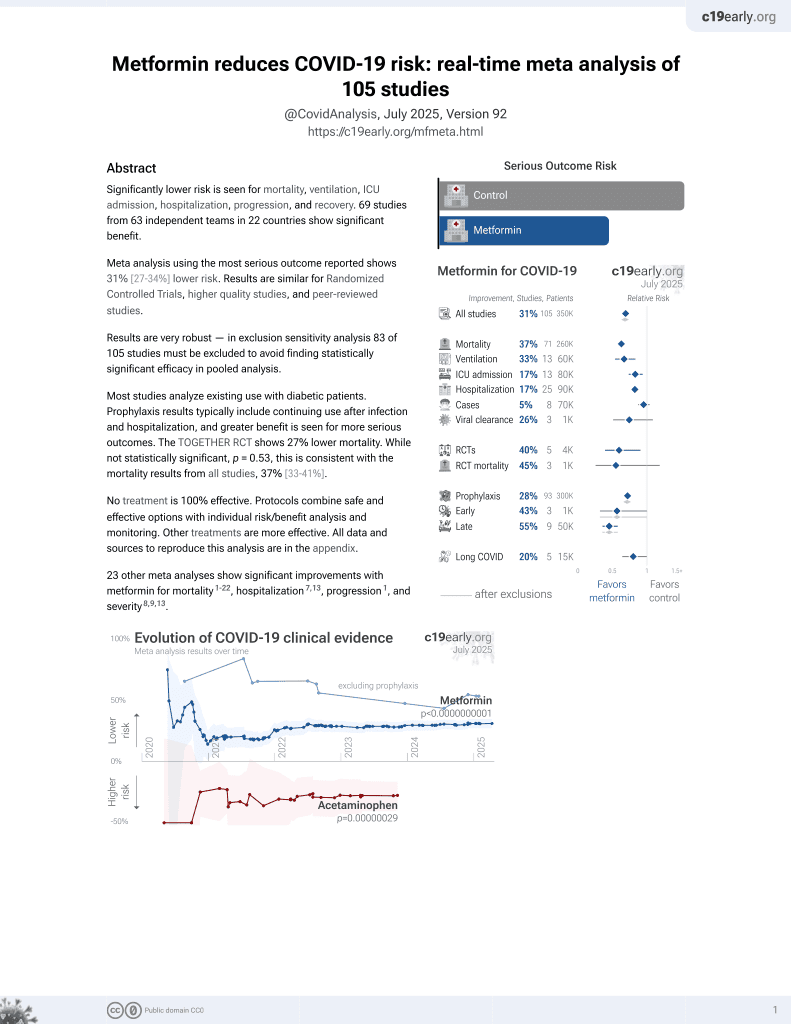
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
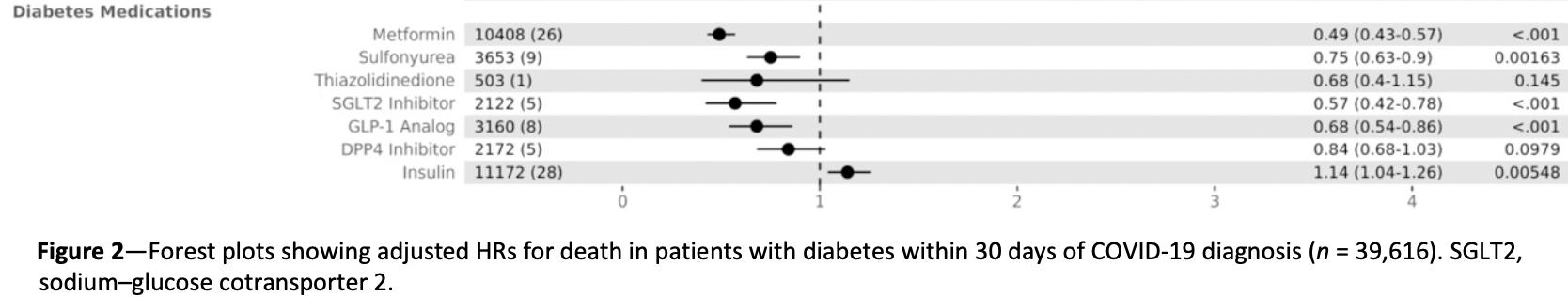
N3C retrospective 39,616 COVID-19 patients with diabetes in the USA, showing lower mortality, ventilation, and hospitalization with metformin use.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 51.0% lower, HR 0.49, p < 0.001, treatment 10,408, control 29,208, Cox proportional hazards.
|
|
risk of mechanical ventilation, 41.0% lower, OR 0.59, p < 0.001, treatment 10,408, control 29,208, adjusted per study, multivariable, RR approximated with OR.
|
|
risk of hospitalization, 40.0% lower, OR 0.60, p < 0.001, treatment 10,408, control 29,208, adjusted per study, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wong et al., 24 Feb 2022, retrospective, USA, peer-reviewed, 15 authors.
Abstract: Diabetes Care
Rachel Wong,1 Margaret Hall,1
Rohith Vaddavalli,2 Adit Anand,1
Neha Arora,3 Carolyn T. Bramante,4
Victor Garcia,1 Steven Johnson,5
Mary Saltz,1 Jena S. Tronieri,6
Yun Jae Yoo,1 John B. Buse,7,8 Joel Saltz,1
Joshua Miller,3 and Richard Moffitt,1
for the N3C Consortium*
OBJECTIVE
The purpose of the study is to evaluate the relationship between HbA1c and
severity of coronavirus disease 2019 (COVID-19) outcomes in patients with type 2
diabetes (T2D) with acute COVID-19 infection.
RESEARCH DESIGN AND METHODS
We conducted a retrospective study using observational data from the National
COVID Cohort Collaborative (N3C), a longitudinal, multicenter U.S. cohort of
patients with COVID-19 infection. Patients were ‡18 years old with T2D and confirmed COVID-19 infection by laboratory testing or diagnosis code. The primary
outcome was 30-day mortality following the date of COVID-19 diagnosis. Secondary outcomes included need for invasive ventilation or extracorporeal membrane
oxygenation (ECMO), hospitalization within 7 days before or 30 days after
COVID-19 diagnosis, and length of stay (LOS) for patients who were hospitalized.
RESULTS
The study included 39,616 patients (50.9% female, 55.4% White, 26.4% Black or
African American, and 16.1% Hispanic or Latino, with mean ± SD age 62.1 ± 13.9
years and mean ± SD HbA1c 7.6% ± 2.0). There was an increasing risk of hospitalization with incrementally higher HbA1c levels, but risk of death plateaued at
HbA1c >8%, and risk of invasive ventilation or ECMO plateaued >9%. There was
no significant difference in LOS across HbA1c levels.
CONCLUSIONS
1
Department of Biomedical Informatics, Stony
Brook University, Stony Brook, NY
2
Department of Computer Science, Stony Brook
University, Stony Brook, NY
3
Division of Endocrinology and Metabolism,
Department of Medicine, Renaissance School
of Medicine at Stony Brook University, Stony
Brook, NY
4
Division of General Internal Medicine,
University of Minnesota Medical School,
Minneapolis, MN
5
Institute for Health Informatics, University of
Minnesota, Minneapolis, MN
6
Department of Psychiatry, Perelman School of
Medicine at the University of Pennsylvania,
Philadelphia, PA
7
Division of Endocrinology and Metabolism,
Department of Medicine, University of North
Carolina School of Medicine, Chapel Hill, NC
8
North Carolina Translational and Clinical
Sciences Institute, University of North Carolina
School of Medicine, Chapel Hill, NC
Corresponding author: Rachel Wong, rachel.
wong@stonybrookmedicine.edu
Received 20 October 2021 and accepted 28
January 2022
This article contains supplementary material online
at https://doi.org/10.2337/figshare.19119302.
In a large, multicenter cohort of patients in the U.S. with T2D and COVID-19 infection, risk of hospitalization increased with incrementally higher HbA1c levels. Risk
of death and invasive ventilation also increased but plateaued at different levels
of glycemic control.
J.M. and R.M. contributed equally.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has claimed >4 million lives worldwide since the first reported case of coronavirus disease-2019
(COVID-19) in December 2019 (1). Diabetes has been implicated as a risk factor for
increased mortality and morbidity in patients with COVID-19 infection, with a
higher prevalence of diabetes reported in patients with severe outcomes, including
© 2022 by the American Diabetes Association.
Readers may use this article as long as the
work is properly..
DOI record:
{
"DOI": "10.2337/dc21-2186",
"ISSN": [
"0149-5992",
"1935-5548"
],
"URL": "http://dx.doi.org/10.2337/dc21-2186",
"abstract": "<jats:sec>\n <jats:title>OBJECTIVE</jats:title>\n <jats:p>The purpose of the study is to evaluate the relationship between HbA1c and severity of coronavirus disease 2019 (COVID-19) outcomes in patients with type 2 diabetes (T2D) with acute COVID-19 infection.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>RESEARCH DESIGN AND METHODS</jats:title>\n <jats:p>We conducted a retrospective study using observational data from the National COVID Cohort Collaborative (N3C), a longitudinal, multicenter U.S. cohort of patients with COVID-19 infection. Patients were ≥18 years old with T2D and confirmed COVID-19 infection by laboratory testing or diagnosis code. The primary outcome was 30-day mortality following the date of COVID-19 diagnosis. Secondary outcomes included need for invasive ventilation or extracorporeal membrane oxygenation (ECMO), hospitalization within 7 days before or 30 days after COVID-19 diagnosis, and length of stay (LOS) for patients who were hospitalized.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>RESULTS</jats:title>\n <jats:p>The study included 39,616 patients (50.9% female, 55.4% White, 26.4% Black or African American, and 16.1% Hispanic or Latino, with mean ± SD age 62.1 ± 13.9 years and mean ± SD HbA1c 7.6% ± 2.0). There was an increasing risk of hospitalization with incrementally higher HbA1c levels, but risk of death plateaued at HbA1c &gt;8%, and risk of invasive ventilation or ECMO plateaued &gt;9%. There was no significant difference in LOS across HbA1c levels.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>CONCLUSIONS</jats:title>\n <jats:p>In a large, multicenter cohort of patients in the U.S. with T2D and COVID-19 infection, risk of hospitalization increased with incrementally higher HbA1c levels. Risk of death and invasive ventilation also increased but plateaued at different levels of glycemic control.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3108-7324",
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"authenticated-orcid": false,
"family": "Wong",
"given": "Rachel",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"family": "Hall",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Computer Science, Stony Brook University, Stony Brook, NY"
}
],
"family": "Vaddavalli",
"given": "Rohith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"family": "Anand",
"given": "Adit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Endocrinology and Metabolism, Department of Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY"
}
],
"family": "Arora",
"given": "Neha",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5858-2080",
"affiliation": [
{
"name": "Division of General Internal Medicine, University of Minnesota Medical School, Minneapolis, MN"
}
],
"authenticated-orcid": false,
"family": "Bramante",
"given": "Carolyn T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"family": "Garcia",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute for Health Informatics, University of Minnesota, Minneapolis, MN"
}
],
"family": "Johnson",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"family": "Saltz",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Psychiatry, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA"
}
],
"family": "Tronieri",
"given": "Jena S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"family": "Yoo",
"given": "Yun Jae",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Endocrinology and Metabolism, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC"
},
{
"name": "North Carolina Translational and Clinical Sciences Institute, University of North Carolina School of Medicine, Chapel Hill, NC"
}
],
"family": "Buse",
"given": "John B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"family": "Saltz",
"given": "Joel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9512-461X",
"affiliation": [
{
"name": "Division of Endocrinology and Metabolism, Department of Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY"
}
],
"authenticated-orcid": false,
"family": "Miller",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY"
}
],
"family": "Moffitt",
"given": "Richard",
"sequence": "additional"
}
],
"container-title": [
"Diabetes Care"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"diabetesjournals.org"
]
},
"created": {
"date-parts": [
[
2022,
2,
24
]
],
"date-time": "2022-02-24T18:38:42Z",
"timestamp": 1645727922000
},
"deposited": {
"date-parts": [
[
2022,
3,
13
]
],
"date-time": "2022-03-13T11:39:59Z",
"timestamp": 1647171599000
},
"indexed": {
"date-parts": [
[
2022,
3,
14
]
],
"date-time": "2022-03-14T09:40:56Z",
"timestamp": 1647250856371
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0149-5992"
},
{
"type": "electronic",
"value": "1935-5548"
}
],
"issued": {
"date-parts": [
[
2022,
2,
24
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.diabetesjournals.org/journals/pages/license",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
24
]
],
"date-time": "2022-02-24T00:00:00Z",
"timestamp": 1645660800000
}
}
],
"link": [
{
"URL": "https://diabetesjournals.org/care/article-pdf/doi/10.2337/dc21-2186/670185/dc212186.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://diabetesjournals.org/care/article-pdf/doi/10.2337/dc21-2186/670185/dc212186.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1167",
"original-title": [],
"prefix": "10.2337",
"published": {
"date-parts": [
[
2022,
2,
24
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
24
]
]
},
"publisher": "American Diabetes Association",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://diabetesjournals.org/care/article/doi/10.2337/dc21-2186/144605/Glycemic-Control-and-Clinical-Outcomes-in-U-S"
}
},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Advanced and Specialized Nursing",
"Endocrinology, Diabetes and Metabolism",
"Internal Medicine"
],
"subtitle": [],
"title": [
"Glycemic Control and Clinical Outcomes in U.S. Patients With COVID-19: Data From the National COVID Cohort Collaborative (N3C) Database"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.2337/ada-journal-policies"
}