Approval delays in multi-country COVID-19 trials: the case of COPCOV and the risk of therapeutic inertia
et al., Trials, doi:10.1186/s13063-025-09300-z, Dec 2025
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 423 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
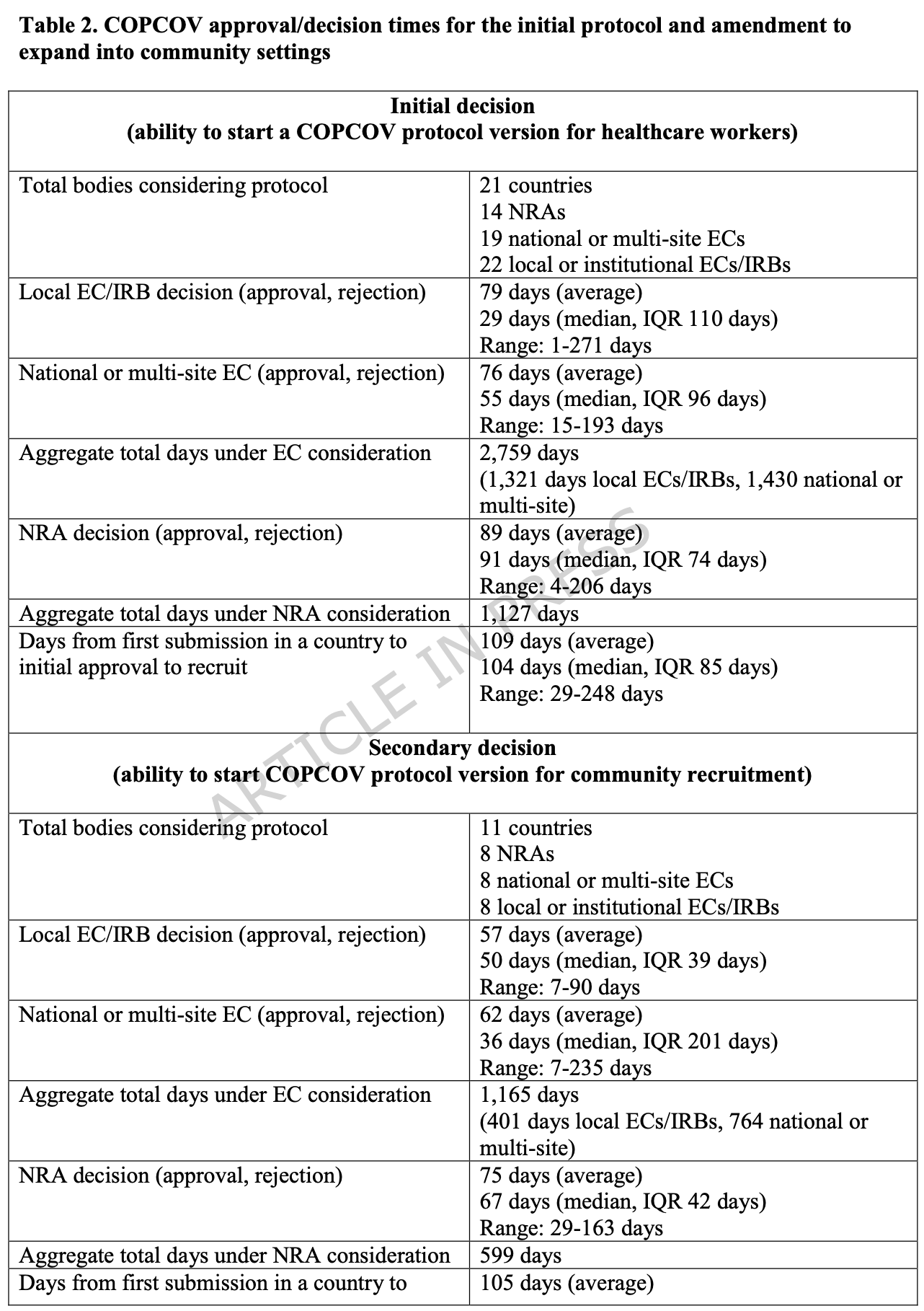
Analysis of bureaucratic approval delays in the multi-country COVID-19 COPCOV trial, showing a median decision time of 104 days across 76 countries, despite expedited emergency review processes. The trial missed critical early COVID-19 waves due to these delays, recruiting less than 5,000 of the planned 40,000 healthcare workers. Sequential rather than parallel reviews, lack of transparency in decision-making, and inflexibility in risk assessment were identified as major barriers. The fraudulent Mehra et al. Lancet paper linking HCQ to cardiotoxicity, though quickly retracted, caused additional months-long delays across multiple countries. Authors argue these approval delays represent a "risk of therapeutic inertia" where bureaucratic risk-aversion overlooks the potential harms of delaying potentially beneficial treatments during health emergencies. The study highlights how existing frameworks for expedited pandemic research approvals largely failed in practice, with even WHO Africa's AVAREF system taking 50+ days instead of the promised 10 days for approvals.
Winters et al., 16 Dec 2025, peer-reviewed, 2 authors.
Contact: janelle.winters@history.ox.ac.uk.
Approval delays in multi-country COVID-19 trials: the case of COPCOV and the risk of therapeutic inertia
Trials, doi:10.1186/s13063-025-09300-z
Background: Many multi-country COVID-19 clinical trials, including those for widely available repurposed drugs with strong safety profiles, were conceptualised quickly but were unable to influence clinical treatment guidelines. The Chloroquine/Hydroxychloroquine for the Prevention of COVID-19 (COPCOV) trial, a large multi-country clinical trial sponsored by the University of Oxford, sought to determine the efficacy of hydroxychloroquine and chloroquine as a prophylaxis for COVID-19, but faced approval delays and other bureaucratic challenges. Understanding the reasons for these delays will help to guide reform for future multi-country trials responding to health emergencies.
Methods: Using an extensive case study of the COPCOV trial, we aimed to quantitatively and qualitatively analyse the bureaucratic challenges facing academic researchers seeking trial approval across multiple countries during health emergencies. We measured the median time from first COPCOV trial protocol submission to an ethics/regulatory body in each country to first approval and disaggregated the average and median time for approval by ethics committees and regulatory bodies. These data are extracted from official documents in the Trial Master File, records from country investigators, and thousands of stakeholder emails. Additionally, we conducted semi-structured interviews with 65 trial stakeholders to identify barriers to approval and we analysed these interviews using inductive thematic analysis. Results: For the COPCOV trial, investigators sought approval in 76 countries and submitted initial protocols to 22 local/institutional ethics committees or institutional research boards, 19 multi-site or national ethics committees, and 14 national regulatory authorities. The median time for the study to receive an initial decision (approval or rejection) in each country was 104 days (IQR 42). Approximately half of the countries to which the COPCOV protocol was submitted had sequential systems for ethics and regulatory review, and those with an
A R T I C L E I N P R E S S Diabetes: A Systematic Review. Diabetes, Obesity and Metabolism. 2018;20 (2) :427-37. [85] Whitney SN, Schneider CE. A Method to Estimate the Cost in Lives of Ethics Board Review of Biomedical Research. Journal of Internal Medicine. 2011;269(4):396-402. [86] London AJ, Kimmelman J. Against Pandemic Research Exceptionalism. Science. 2020;368(6490):476-7.
List of abbreviations
Declarations
Ethics approval and consent to participate All interviews were conducted by JW with approval from the University of Oxford"s CUREC, reference R81146/RE001 and stakeholders" identities were anonymised based on consent preferences.
Consent for publication All authors consent to publication of this manuscript.
Availability of data and materials
A R T I C L E I N P R E S S The datasets generated and analysed during the current study are not publicly available, in compliance with GDPR and anonymisation as per the University of Oxford"s ethics clearance (CUREC) requirements. However, these data are available on reasonable request from the authors, subject to the removal of any personally identifying information, as are more detailed primary document bibliographies.
Competing interests None declared.
Authors' contributions JW compiled the quantitative dataset, conducted stakeholder interviews, performed initial data and thematic analysis, drafted the initial version of the manuscript, and led revisions. WS is a co-principal investigator of..
References
A R T I C L E I N P R E S S ; Horby, COVID-19 Clinical Trials: From Concept to Results, COVID-19 Updates from Singapore
A R T I C L E I N P R E S S ; Jacobs, Ljungberg, How Ethics Travels: The International Development of Research Ethics Committees in the Late Twentieth Century, European Journal for the History of Medicine and Health
A R T I C L E I N P R E S S ; White, Cardiotoxicity of antimalarial drugs, The Lancet infectious diseases
A R T I C L E I N P R E S S, None
A R T I C L E I N P R E S S, None
A R T I C L E I N P R E S S, None
A R T I C L E I N P R E S S, None, doi:10.1177/20563051211024977
A R T I C L E I N P R E S S, World Health Organization. First WHO Global Clinical Trials Forum Puts Forward a Global Vision for Sustainable Clinical Research Infrastructure
Abraham, The Pharmaceutical Industry as a Political Player, The Lancet
Akanmori, Mukanga, Bellah, Traore, Ward et al., The Role of the African Vaccine Regulatory Forum (AVAREF) in the Accelerated Clinical Evaluation of Ebola Vaccine Candidates During the Large West African Epidemic, Journal of Immunological Sciences
Alirol, Kuesel, Guraiib, De La Fuente-Nunez, Saxena et al., Ethics Review of Studies During Public Health Emergencies -The Experience of the WHO Ethics Review Committee during the Ebola Virus Disease Epidemic, BMC Medical Ethics, doi:.org/10.1186/s12910-017-0201-1
Amuasi, Research Readiness for Response as a Foundation for Effective Research in Global Public Health Emergencies (Panel Presentation)
Annane, Antona, Lehmann, Kedzia, Chevret, Designing and Conducting a Randomized Trial for Pandemic Critical Illness: The 2009 H1N1 Influenza Pandemic, Intensive Care Medicine
Blevins, Edgerton, Lee, Shouting into the Wind: Medical Science versus "BS" in the Twitter Maelstrom of Politics and Misinformation About Hydroxychloroquine, Social Media & Society, doi:10.1177/20563051211024977
Boetto, Golinelli, Carullo, Fantini, Frauds in Scientific Research and How to Possibly Overcome Them, Journal of Medical Ethics
Braun, Clarke, Using Thematic Analysis in Psychology, Qualitative Research in Psychology
Cavaleri, De Sousa, Hacker, Higgs, Lumpkin et al., A Roadmap for Fostering Timely Regulatory and Ethics Approvals of International Clinical Trials in Support of Global Health Research Systems, The Lancet Global Health
Chattopadhyay, Mahajan, Kumar, Assessment of Safety of the Major Antimalarial Drugs, Expert Opinion on Drug Safety
Copcov Dsmc, None
Crosby, Malavisi, Huang, Holden, Neal, Factors Influencing the Time to Ethics and Governance Approvals for Clinical Trials: A Retrospective Cross-Sectional Survey, Trials
Davey, Kirchgaessner, Surgisphere: Mass Audit of Papers Linked to Firm Behind Hydroxychloroquine Lancet Study Scandal, The Guardian
Diallo, Trøseid, Simensen, Boston, Demotes et al., Accelerating Clinical Trial Implementation in the Context of the COVID-19 Pandemic: Challenges, Lessons Learned and A R T I C L E I N P R E S S Recommendations from DisCoVeRy and the EU-SolidAct EU Response Group, Clinical Microbiology and Infection
Gershkovich, Sauer, As Russian Officials Back Hydroxychloroquine, Doctors Take Matters Into their Own Hands, The Moscow Times
Glasziou, Scott, Chalmers, Kolstoe, Davies, Improving Research Ethics Review and Governance Can Improve Human Health, Journal of the Royal Society of Medicine
Gobat, Amuasi, Yazdanpanah, Sigfid, Davies et al., Advancing Preparedness for Clinical Research During Infectious Disease Epidemics, ERJ Open Research, doi:10.1183/23120541.00227-2018
Goossens, Derde, Horby, Bonten, The European Clinical Research Response to Optimise Treatment of Patients with COVID-19: Lessons Learned, Future Perspective, and Recommendations, The Lancet Infectious Diseases
Gumber, Agbeleye, Inskip, Fairbairn, Still et al., Operational Complexities in International Clinical trials: A Systematic Review of Challenges and Proposed Solutions, BMJ Open
Haeusler, Chan, Guérin, White, The Arrhythmogenic Cardiotoxicity of the Quinoline and Structurally Related Antimalarial Drugs: A Systematic Review, BMC medicine, doi:.org/10.1186/s12916-018-1188-2
Halpern, Karlawish, Berlin, The Continuing Unethical Conduct of Underpowered Clinical Trials, JAMA
Harris, Associates, Strengthening Regulatory Systems in LMICs: Improving the Sustainability of the Vaccine Innovation Ecosystem in Africa
Harris, Associates, Strengthening Regulatory Systems in LMICs: Improving the Sustainability of the Vaccine Innovation Ecosystem in Africa
Hinga, Jeena, Awuor, Kahindi, Murene et al., Pandemic Preparedness and Responsiveness of Research Review Committees: Lessons from Review of COVID-19 Protocols at KEMRI Wellcome Trust Research Programme in Kenya, Wellcome Open Research, doi:10.12688/wellcomeopenres.17533.2
Holthof, White, Fwd: chloroquine and hydroxychloroquine trial can prevent COVID-19
Jones, Adams, Murphy, King, Saracco et al., Delays in Reporting and Publishing Trial Results During Pandemics: Cross Sectional Analysis of 2009 H1N1, 2014 Ebola, and 2016 Zika Clinical Trials, BMC Medical Research Methodology, doi:.org/10.1186/s12874-021-01324-8
Khunti, Gomes, Pocock, Shestakova, Pintat et al., Therapeutic Inertia in the Treatment of Hyperglycaemia in Patients with Type 2
Llewelyn, Milton, Adler, Dougall, Chalk, Chloroquine/ hydroxychloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting; a randomised, placebo-controlled prophylaxis study, COPCOV)
Mak, Lim, Thanaphollert, Mahlangu, Cooke et al., Global Regulatory Agility During COVID-19 and Other Health Emergencies, BMJ, doi:.org/10.1136/bmj.m1575
Matangila, ANTICOV: Initiating a Platform Adaptive Trial for COVID Outpatients in Africa, Journal of Public Health in Africa
Mehra, Ruschitzka, Patel, Retraction: Hydroxychloroquine or Chloroquine with or without a Macrolide for Treatment of COVID-19: A Multinational Registry Analysis, The Lancet, doi:.org/10.1016/S0140-6736(20)31324-6
Nakada, Hasthorpe, Ijsselmuiden, Kombe, Ba et al., Recommendations for Promoting International Multi-Site Clinical Trials: From a Viewpoint of Ethics Review, Developing World Bioethics
Ndebele, Blanchard-Horan, Shahkolahi, Sanne, Regulatory Challenges Associated with Conducting Multi-Country Clinical Trials in Resource-Limited Settings, Journal of Acquired Immune Deficiency Syndromes
Ndebele, Blanchard-Horan, Shahkolahi, Sanne, Regulatory Challenges Associated with Conducting Multi-Country Clinical Trials in Resource-Limited Settings, Journal of Acquired Immune Deficiency Syndromes
Peiffer-Smadja, Rebeaud, Guihur, Mahamat-Saleh, Fiolet, Hydroxychloroquine and COVID-19: A Tale of Populism and Obscurantism, The Lancet Infectious Diseases, doi:.org/10.1016/S1473-3099(20)30866-5
Petryna, When Experiments Travel: Clinical Trials and the Global Search for Human Subjects
Rid, Emanuel, Wendler, Evaluating the Risks of Clinical Research, JAMA
Saxena, Horby, Amuasi, Aagaard, Köhler et al., Ethics Preparedness: Facilitating Ethics Review During Outbreaks: Recommendations from an Expert Panel, BMC Medical Ethics, doi:.org/10.1186/s12910-019-0366-x
Schilling, Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus, PubMed -NCBI
Schilling, Mukaka, Callery, Llewelyn, Cruz et al., Evaluation of Hydroxychloroquine or Chloroquine for the Prevention of COVID-19 (COPCOV): A Double-blind, Randomised, Placebo-Controlled Trial, PLoS Medicine
Schilling, White, Does Hydroxychloroquine Still Have Any Role in the COVID-19 Pandemic?, Expert Opinion on Pharmacotherapy
Schopper, Ravinetto, Schwartz, Kamaara, Sheel et al., Research Ethics Governance in Times of Ebola, Public Health Ethics
Shah, Safri, Thevadas, Noordin, Rahman et al., COVID-19 Outbreak in Malaysia: Actions Taken by the Malaysian government, International Journal of Infectious Diseases
Sigfrid, Maskell, Bannister, Ismail, Collinson et al., Addressing Challenges for Clinical Research Responses to Emerging Epidemics and Pandemics: A Scoping Review, BMC Medicine, doi:.org/10.1186/s12916-020-01624-8
Simpson, Chakrabarti, Robinson, Chirgwin, Lumpkin, Navigating Facilitated Regulatory Pathways During a Disease X Pandemic, NPI Vaccines, doi:.org/10.1038/s41541-020-00249-5
Simpson, Chakrabarti, Robinson, Chirgwin, Lumpkin, Navigating Facilitated Regulatory Pathways During a Disease X Pandemic, npj vaccines, doi:.org/10.1038/s41541-020-00249-5
Tass, Russia Removes Hydroxychloroquine from List of Drugs Used to Treat COVID-19, Russia News Agency
Td, Bourner, Kali, Trøseid, Yazdanpanah et al., Experiences and challenges with the New European Clinical Trials Regulation, Trials
Ting, A Strategic Theory of Bureaucratic Redundancy, American Journal of Political Science
Tusino, Furfaro, Rethinking the Role of Research Ethics Committees in the Light of Regulation (EU) No 536/2014 on Clinical Trials and the COVID-19 Pandemic, British Journal of Clinical Pharmacology
Vincent, Bergeron, Benjannet, Erickson, Rollin et al., Chloroquine is a Potent Inhibitor of SARS Coronavirus Infection and Spread, Virology Journal, doi:.org/10.1186/1743-422X-2-69
Vissers, Cohen, Van Gerven, Groeneveld, The Impact of the Global COVID-19 Pandemic on the Conduct of Clinical Trials: Return to Normalcy by Considering the Practical Impact of a Structured Ethical Analysis, British Journal of Clinical Pharmacology
Wamai, Shirley, The Future of Health in Sub-Saharan Africa: Is There a Path to Longer and Healthier Lives for All?
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-nCoV) in vitro, Cell Research
White, Clinical Trials in Tropical Diseases: A Politically Incorrect View, Tropical Medicine & International Health
White, Horby, Chloroquine for Novel Coronavirus? 10
Who, WHO Tool for Benchmarking Ethics Oversight of Health-Related Research with Human Participants
Wise, Coombes, COVID-19: The Inside Story of the RECOVERY Trial, BMJ, doi:.org/10.1136/bmj.m2670
Yarborough, Do We Really Know How Many Clinical Trials Are Conducted Ethically? Why Research Ethics Committee Review Practices Need to be Strengthened and Initial Steps We Could Take to Strengthen Them, Journal of Medical Ethics
DOI record:
{
"DOI": "10.1186/s13063-025-09300-z",
"ISSN": [
"1745-6215"
],
"URL": "http://dx.doi.org/10.1186/s13063-025-09300-z",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Many multi-country COVID-19 clinical trials, including those for widely available repurposed drugs with strong safety profiles, were conceptualised quickly but were unable to influence clinical treatment guidelines. The Chloroquine/Hydroxychloroquine for the Prevention of COVID-19 (COPCOV) trial, a large multi-country clinical trial sponsored by the University of Oxford, sought to determine the efficacy of hydroxychloroquine and chloroquine as a prophylaxis for COVID-19 but faced approval delays and other bureaucratic challenges. Understanding the reasons for these delays will help to guide reform for future multi-country trials responding to health emergencies.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Using an extensive case study of the COPCOV trial, we aimed to quantitatively and qualitatively analyse the bureaucratic challenges facing academic researchers seeking trial approval across multiple countries during health emergencies. We measured the median time from first COPCOV trial protocol submission to an ethics/regulatory body in each country to first approval and disaggregated the average and median time for approval by ethics committees and regulatory bodies. These data are extracted from official documents in the Trial Master File, records from country investigators, and thousands of stakeholder emails. Additionally, we conducted semi-structured interviews with 65 trial stakeholders to identify barriers to approval, and we analysed these interviews using inductive thematic analysis.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>\n For the COPCOV trial, investigators sought approval in 76 countries and submitted initial protocols to 22 local/institutional ethics committees or institutional research boards, 19 multisite or national ethics committees, and 14 national regulatory authorities. The median time for the study to receive an initial decision (approval or rejection) in each country was 104 days (\n <jats:italic>IQR</jats:italic>\n 42). Approximately half of the countries to which the COPCOV protocol was submitted had sequential systems for ethics and regulatory review, and those with an expedited review system communicated faster decisions (median 91 days vs. 122 days). Issues with efficiency, flexibility, and decision-making coherence underpinned these approval delays. Efficiency challenges included overlap in comments between ethics bodies and duplicative ethics and regulatory body roles. Delays due to inflexibility resulted from under-awareness of existing risk-based frameworks for repurposed drugs, few mechanisms for streamlining documentation requirements during emergency review processes, and under-utilisation of regulatory agility and reliance mechanisms. Objectivity and coherence of decision-making by trial approval bodies were limited by a lack of stringent regulatory authority transparency and limited communication channels between trial stakeholders.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Trial approval challenges are rooted in a combination of conservative good clinical practice interpretation and insufficient international guidance and leadership, which contribute to a dangerous ‘risk of therapeutic inertia’ in developing evidence during public health emergencies. Governance reforms to address these challenges should be twofold, focused on improving national awareness, buy-in, and financing for existing harmonisation and risk-based structures and establishing a global framework for clinical research during health emergencies.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>ClinicalTrials.gov NCT04303507. Registered on 11 March 2020.</jats:p>\n </jats:sec>",
"alternative-id": [
"9300"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "23 September 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 November 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "16 December 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "All interviews were conducted by J. W. with approval from the University of Oxford’s CUREC, reference R81146/RE001, and stakeholders’ identities were anonymised based on consent preferences."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "All authors consent to publication of this manuscript."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-3450-7673",
"affiliation": [],
"authenticated-orcid": false,
"family": "Winters",
"given": "Janelle",
"sequence": "first"
},
{
"affiliation": [],
"family": "Schilling",
"given": "William HK",
"sequence": "additional"
}
],
"container-title": "Trials",
"container-title-short": "Trials",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
12,
16
]
],
"date-time": "2025-12-16T04:34:26Z",
"timestamp": 1765859666000
},
"deposited": {
"date-parts": [
[
2025,
12,
16
]
],
"date-time": "2025-12-16T04:34:36Z",
"timestamp": 1765859676000
},
"funder": [
{
"DOI": "10.13039/100010269",
"award": [
"221307/Z/20/Z"
],
"award-info": [
{
"award-number": [
"221307/Z/20/Z"
]
}
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100010269",
"id-type": "DOI"
}
],
"name": "Wellcome Trust"
}
],
"indexed": {
"date-parts": [
[
2025,
12,
16
]
],
"date-time": "2025-12-16T04:36:25Z",
"timestamp": 1765859785080,
"version": "3.48.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
12,
16
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
12,
16
]
],
"date-time": "2025-12-16T00:00:00Z",
"timestamp": 1765843200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
12,
16
]
],
"date-time": "2025-12-16T00:00:00Z",
"timestamp": 1765843200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-025-09300-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13063-025-09300-z/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-025-09300-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
12,
16
]
]
},
"published-online": {
"date-parts": [
[
2025,
12,
16
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "9300_CR1",
"unstructured": "Schilling W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. – PubMed – NCBI. Sent to White N. 26 January 2020."
},
{
"key": "9300_CR2",
"unstructured": "Mahidol Oxford Tropical Medicine Research Unit. COPCOV Living Daily. 26 May 2022."
},
{
"key": "9300_CR3",
"unstructured": "Mahidol Oxford Tropical Medicine Research Unit. COPCOV now world’s largest COVID-19 pre-exposure prophylaxis trial. Available from: https://www.tropmedres.ac/news/copcov-now-world2019s-largest-covid-19-pre-exposure-prophylaxis-trial. Accessed 2 Feb 2024."
},
{
"key": "9300_CR4",
"unstructured": "Bruno Holthof to Nick White. Fwd: chloroquine and hydroxychloroquine trial can prevent COVID-19. 21 May 2020."
},
{
"DOI": "10.1186/1743-422X-2-69",
"author": "MJ Vincent",
"doi-asserted-by": "publisher",
"journal-title": "Virol J",
"key": "9300_CR5",
"unstructured": "Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005. https://doi.org/10.1186/1743-422X-2-69.",
"year": "2005"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"author": "M Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "3",
"journal-title": "Cell Res",
"key": "9300_CR6",
"unstructured": "Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–71.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1186/s12916-018-1188-2",
"author": "IL Haeusler",
"doi-asserted-by": "publisher",
"journal-title": "BMC Med",
"key": "9300_CR7",
"unstructured": "Haeusler IL, Chan XH, Guérin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018. https://doi.org/10.1186/s12916-018-1188-2.",
"year": "2018"
},
{
"DOI": "10.1016/S1473-3099(07)70187-1",
"author": "NJ White",
"doi-asserted-by": "crossref",
"first-page": "549",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "9300_CR8",
"unstructured": "White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–58.",
"volume": "7",
"year": "2007"
},
{
"key": "9300_CR9",
"unstructured": "Nick White to Peter Horby. Chloroquine for novel coronavirus? 10 February 2020."
},
{
"DOI": "10.1517/14740338.6.5.505",
"author": "R Chattopadhyay",
"doi-asserted-by": "crossref",
"first-page": "505",
"issue": "5",
"journal-title": "Expert Opin Drug Saf",
"key": "9300_CR10",
"unstructured": "Chattopadhyay R, Mahajan B, Kumar S. Assessment of safety of the major antimalarial drugs. Expert Opin Drug Saf. 2007;6(5):505–21.",
"volume": "6",
"year": "2007"
},
{
"DOI": "10.1186/ISRCTN10207947",
"doi-asserted-by": "publisher",
"key": "9300_CR11",
"unstructured": "Mahidol-Oxford Tropical Medicine Research Unit. Study of chloroquine/hydroxychloroquine and coronavirus disease (COVID-19) in the healthcare setting. ISRCTNregistry. 2020;ISCRCTN10207947:doi.org/https://doi.org/10.1186/ISRCTN10207947."
},
{
"DOI": "10.1136/bmj.m2670",
"doi-asserted-by": "publisher",
"key": "9300_CR12",
"unstructured": "Wise J, Coombes R. COVID-19: the inside story of the RECOVERY trial. BMJ 2020; 370(m2670):doi.org/https://doi.org/10.1136/bmj.m2670."
},
{
"key": "9300_CR13",
"unstructured": "Llewelyn M to Milton J, Adler A, Dougall G, Chalk J. Chloroquine/ hydroxychloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting; a randomised, placebo-controlled prophylaxis study (COPCOV). 22 May 2020."
},
{
"DOI": "10.1016/S1473-3099(21)00705-2",
"author": "H Goossens",
"doi-asserted-by": "crossref",
"first-page": "e153",
"issue": "5",
"journal-title": "Lancet Infect Dis",
"key": "9300_CR14",
"unstructured": "Goossens H, Derde L, Horby P, Bonten M. The European clinical research response to optimise treatment of patients with COVID-19: lessons learned, future perspective, and recommendations. Lancet Infect Dis. 2022;22(5):e153-8.",
"volume": "22",
"year": "2022"
},
{
"author": "WHK Schilling",
"issue": "9",
"journal-title": "PLoS Med",
"key": "9300_CR15",
"unstructured": "Schilling WHK, Mukaka M, Callery JJ, Llewelyn MJ, Cruz CV, Dhorda M, et al. Evaluation of hydroxychloroquine or chloroquine for the prevention of COVID-19 (COPCOV): a double-blind, randomised, placebo-controlled trial. PLoS Med. 2024;21(9):e1004428.",
"volume": "21",
"year": "2024"
},
{
"key": "9300_CR16",
"unstructured": "Horby P. COVID-19 clinical trials: from concept to results, COVID-19 Updates from Singapore, Presentation at the NUS Yong Loo Lin School of Medicine, National University of Health System. 15 August 2020. Available from: https://medicine.nus.edu.sg/news/covid-19-clinical-trials-from-concept-to-results/."
},
{
"DOI": "10.29245/2578-3009/2018/si.1111",
"doi-asserted-by": "crossref",
"key": "9300_CR17",
"unstructured": "Akanmori DB, Mukanga D, Bellah A, Traore T, Ward M, Mihigo R. The role of the African Vaccine Regulatory Forum (AVAREF) in the accelerated clinical evaluation of Ebola vaccine candidates during the large West African epidemic. J Immunol Sci. 2018;S(001):75–79."
},
{
"DOI": "10.1097/QAI.0000000000000037",
"author": "P Ndebele",
"doi-asserted-by": "crossref",
"first-page": "S29",
"issue": "01",
"journal-title": "J Acquir Immune Defic Syndr",
"key": "9300_CR18",
"unstructured": "Ndebele P, Blanchard-Horan C, Shahkolahi A, Sanne I. Regulatory challenges associated with conducting multi-country clinical trials in resource-limited settings. J Acquir Immune Defic Syndr. 2014;65(01):S29-31.",
"volume": "65",
"year": "2014"
},
{
"key": "9300_CR19",
"unstructured": "World Health Organization. Guidance for mapping ethical issues in infectious disease outbreaks. 11 July 2016. Available from: https://www.who.int/publications/i/item/guidance-for-managing-ethical-issues-in-infectious-disease-outbreaks."
},
{
"author": "National Academies of Sciences, Engineering and Medicine, Committee on Clinical Trials During the 2014-2015 Ebola Outbreak",
"key": "9300_CR20",
"unstructured": "National Academies of Sciences, Engineering and Medicine, Committee on Clinical Trials During the 2014-2015 Ebola Outbreak. Integrating Clinical Research into Epidemic Response: The Ebola Experience. Washington DC: National Academies Press; 2017.",
"volume-title": "Integrating Clinical Research into Epidemic Response: The Ebola Experience",
"year": "2017"
},
{
"key": "9300_CR21",
"unstructured": "World Health Organization Regional Committee for Africa. Status of reviews, authorizations and oversight for clinical trials in the WHO African region, AFR/RC67/14. 30 August 2017. Available from: https://www.afro.who.int/sites/default/files/2018-02/AFR-RC67-14%20Status%20of%20reviews-authorizations%20Clinical%20Trials_1.pdf."
},
{
"DOI": "10.1007/s00134-011-2409-8",
"author": "D Annane",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Intensive Care Med",
"key": "9300_CR22",
"unstructured": "Annane D, Antona M, Lehmann B, Kedzia C, Chevret S. Designing and conducting a randomized trial for pandemic critical illness: the 2009 H1N1 influenza pandemic. Intensive Care Med. 2012;38:29–39.",
"volume": "38",
"year": "2012"
},
{
"DOI": "10.1186/s12910-017-0201-1",
"author": "E Alirol",
"doi-asserted-by": "publisher",
"journal-title": "BMC Med Ethics",
"key": "9300_CR23",
"unstructured": "Alirol E, Kuesel AC, Guraiib MM, de la Fuente-Nunez V, Saxena A, Gomes MF. Ethics review of studies during public health emergencies – the experience of the WHO Ethics Review Committee during the Ebola virus disease epidemic. BMC Med Ethics. 2017. https://doi.org/10.1186/s12910-017-0201-1.",
"year": "2017"
},
{
"DOI": "10.1111/dewb.12245",
"author": "H Nakada",
"doi-asserted-by": "crossref",
"first-page": "192",
"issue": "4",
"journal-title": "Dev World Bioeth",
"key": "9300_CR24",
"unstructured": "Nakada H, Hasthorpe S, IJsselmuiden C, Kombe F, Ba M, Matei M, et al. Recommendations for promoting international multi‐site clinical trials: from a viewpoint of ethics review. Dev World Bioeth. 2019;19(4):192–5.",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1038/s41541-020-00249-5",
"author": "S Simpson",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "NPJ Vaccines",
"key": "9300_CR25",
"unstructured": "Simpson S, Chakrabarti A, Robinson D, Chirgwin K, Lumpkin M. Navigating facilitated regulatory pathways during a disease X pandemic. NPJ Vaccines. 2020;5(1):101. https://doi.org/10.1038/s41541-020-00249-5.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1183/23120541.00227-2018",
"author": "N Gobat",
"doi-asserted-by": "crossref",
"journal-title": "ERJ Open Res",
"key": "9300_CR26",
"unstructured": "Gobat N, Amuasi J, Yazdanpanah Y, Sigfid L, Davies H, Byrne JP, et al. Advancing preparedness for clinical research during infectious disease epidemics. ERJ Open Res. 2019;5:10.1183/23120541.00227-2018.",
"volume": "5",
"year": "2019"
},
{
"DOI": "10.1186/s12910-019-0366-x",
"author": "A Saxena",
"doi-asserted-by": "publisher",
"journal-title": "BMC Med Ethics",
"key": "9300_CR27",
"unstructured": "Saxena A, Horby P, Amuasi J, Aagaard N, Köhler J, Gooshki ES, et al. Ethics preparedness: facilitating ethics review during outbreaks: recommendations from an expert panel. BMC Med Ethics. 2019;20:29. https://doi.org/10.1186/s12910-019-0366-x.",
"volume": "20",
"year": "2019"
},
{
"key": "9300_CR28",
"unstructured": "Nuffield Council on Bioethics. Research in global health emergencies: ethical issues. London: Nuffield Council on Bioethics, 2020. Available from: https://www.nuffieldbioethics.org/publications/research-in-global-health-emergencies."
},
{
"author": "D Schopper",
"first-page": "49",
"issue": "1",
"journal-title": "Public Health Ethics",
"key": "9300_CR29",
"unstructured": "Schopper D, Ravinetto R, Schwartz L, Kamaara E, Sheel S, Segelid MJ, et al. Research ethics governance in times of Ebola. Public Health Ethics. 2017;10(1):49–61.",
"volume": "10",
"year": "2017"
},
{
"DOI": "10.1186/s12916-020-01624-8",
"author": "L Sigfrid",
"doi-asserted-by": "publisher",
"journal-title": "BMC Med",
"key": "9300_CR30",
"unstructured": "Sigfrid L, Maskell K, Bannister PG, Ismail SA, Collinson S, Regmi S, et al. Addressing challenges for clinical research responses to emerging epidemics and pandemics: a scoping review. BMC Med. 2020;18:190. https://doi.org/10.1186/s12916-020-01624-8.",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1001/jama.288.3.358",
"author": "SD Halpern",
"doi-asserted-by": "crossref",
"first-page": "358",
"issue": "3",
"journal-title": "JAMA",
"key": "9300_CR31",
"unstructured": "Halpern SD, Karlawish JH, Berlin JA. The continuing unethical conduct of underpowered clinical trials. JAMA. 2002;288(3):358–62.",
"volume": "288",
"year": "2002"
},
{
"DOI": "10.1186/s12874-021-01324-8",
"author": "CW Jones",
"doi-asserted-by": "publisher",
"journal-title": "BMC Med Res Methodol",
"key": "9300_CR32",
"unstructured": "Jones CW, Adams AC, Murphy E, King RP, Saracco B, Stesis KR, et al. Delays in reporting and publishing trial results during pandemics: cross sectional analysis of 2009 H1N1, 2014 Ebola, and 2016 Zika clinical trials. BMC Med Res Methodol. 2021. https://doi.org/10.1186/s12874-021-01324-8.",
"year": "2021"
},
{
"DOI": "10.12688/wellcomeopenres.17533.2",
"doi-asserted-by": "publisher",
"key": "9300_CR33",
"unstructured": "Hinga A, Jeena L, Awuor E, Kahindi J, Murene M, Kinyanjui S et al. Pandemic preparedness and responsiveness of Research Review Committees: lessons from review of COVID-19 protocols at KEMRI Wellcome Trust Research Programme in Kenya. Wellcome Open Research. 2022;7(75):https://doi.org/10.12688/wellcomeopenres.17533.2."
},
{
"author": "TD Patrick-Brown",
"issue": "1",
"journal-title": "Trials",
"key": "9300_CR34",
"unstructured": "Patrick-Brown TD, Bourner J, Kali S, Trøseid M, Yazdanpanah Y, Olliaro P, et al. Experiences and challenges with the New European Clinical Trials Regulation. Trials. 2024;25(1):3.",
"volume": "25",
"year": "2024"
},
{
"DOI": "10.1016/j.cmi.2021.10.011",
"author": "A Diallo",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Clin Microbiol Infect",
"key": "9300_CR35",
"unstructured": "Diallo A, Trøseid M, Simensen VC, Boston A, Demotes J, Olsen IC, et al. Accelerating clinical trial implementation in the context of the COVID-19 pandemic: challenges, lessons learned and recommendations from DisCoVeRy and the EU-SolidAct EU response group. Clin Microbiol Infect. 2022;28(1):1–5.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1191/1478088706qp063oa",
"author": "V Braun",
"doi-asserted-by": "crossref",
"first-page": "77",
"issue": "2",
"journal-title": "Qual Res Psychol",
"key": "9300_CR36",
"unstructured": "Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.",
"volume": "3",
"year": "2006"
},
{
"DOI": "10.1016/S0140-6736(20)31324-6",
"doi-asserted-by": "publisher",
"key": "9300_CR37",
"unstructured": "Mehra MR, Ruschitzka F, Patel AN. Retraction: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet. 2020;395(10240):https://doi.org/10.1016/S0140-6736(20)31324-6."
},
{
"author": "M Davey",
"first-page": "10",
"journal-title": "The Guardian",
"key": "9300_CR38",
"unstructured": "Davey M, Kirchgaessner S. Surgisphere: mass audit of papers linked to firm behind hydroxychloroquine Lancet study scandal. The Guardian. 2020Jun;10:10.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1136/medethics-2020-106639",
"author": "E Boetto",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "J Med Ethics",
"key": "9300_CR39",
"unstructured": "Boetto E, Golinelli D, Carullo G, Fantini MP. Frauds in scientific research and how to possibly overcome them. J Med Ethics. 2021;47(12):e19. https://doi.org/10.1136/medethics-2020-106639.",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2023-077132",
"author": "L Gumber",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "BMJ Open",
"key": "9300_CR40",
"unstructured": "Gumber L, Agbeleye O, Inskip A, Fairbairn R, Still M, Ouma L, et al. Operational complexities in international clinical trials: a systematic review of challenges and proposed solutions. BMJ Open. 2024;14(4):e077132. https://doi.org/10.1136/bmjopen-2023-077132.",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1186/s13063-023-07802-2",
"author": "S Crosby",
"doi-asserted-by": "crossref",
"first-page": "779",
"issue": "1",
"journal-title": "Trials",
"key": "9300_CR41",
"unstructured": "Crosby S, Malavisi A, Huang L, Jan S, Holden R, Neal B. Factors influencing the time to ethics and governance approvals for clinical trials: a retrospective cross-sectional survey. Trials. 2023;24(1):779.",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1177/01410768211051711",
"author": "P Glasziou",
"doi-asserted-by": "crossref",
"first-page": "556",
"issue": "12",
"journal-title": "J R Soc Med",
"key": "9300_CR42",
"unstructured": "Glasziou P, Scott AM, Chalmers I, Kolstoe SE, Davies HT. Improving research ethics review and governance can improve human health. J R Soc Med. 2021;114(12):556–62.",
"volume": "114",
"year": "2021"
},
{
"DOI": "10.1111/1540-5907.00019",
"author": "MM Ting",
"doi-asserted-by": "crossref",
"first-page": "274",
"issue": "2",
"journal-title": "Am J Polit Sci",
"key": "9300_CR43",
"unstructured": "Ting MM. A strategic theory of bureaucratic redundancy. Am J Polit Sci. 2003;47(2):274–92.",
"volume": "47",
"year": "2003"
},
{
"DOI": "10.1163/26667711-20210001",
"author": "N Jacobs",
"doi-asserted-by": "crossref",
"first-page": "257",
"issue": "2",
"journal-title": "Eur J Hist Med Health",
"key": "9300_CR44",
"unstructured": "Jacobs N, Ljungberg HT. How ethics travels: the international development of research ethics committees in the late twentieth century. Eur J Hist Med Health. 2021;78(2):257–65.",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.1136/medethics-2019-106014",
"author": "M Yarborough",
"doi-asserted-by": "crossref",
"first-page": "572",
"issue": "8",
"journal-title": "J Med Ethics",
"key": "9300_CR45",
"unstructured": "Yarborough M. Do we really know how many clinical trials are conducted ethically? Why research ethics committee review practices need to be strengthened and initial steps we could take to strengthen them. J Med Ethics. 2021;47(8):572–9.",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1001/jama.2010.1414",
"author": "A Rid",
"doi-asserted-by": "crossref",
"first-page": "1472",
"issue": "13",
"journal-title": "JAMA",
"key": "9300_CR46",
"unstructured": "Rid A, Emanuel EJ, Wendler D. Evaluating the risks of clinical research. JAMA. 2010;304(13):1472–9.",
"volume": "304",
"year": "2010"
},
{
"DOI": "10.1111/bcp.14480",
"author": "MF Vissers",
"doi-asserted-by": "crossref",
"first-page": "837",
"issue": "3",
"journal-title": "Br J Clin Pharmacol",
"key": "9300_CR47",
"unstructured": "Vissers MF, Cohen AF, Van Gerven JM, Groeneveld GJ. The impact of the global COVID‐19 pandemic on the conduct of clinical trials: return to normalcy by considering the practical impact of a structured ethical analysis. Br J Clin Pharmacol. 2021;87(3):837–44.",
"volume": "87",
"year": "2021"
},
{
"key": "9300_CR48",
"unstructured": "Organisation for Economic Cooperation on Development. OECD/LEGAL/0397: Recommendations of the Council on the Governance of Clinical Trials. 2012. Available from: https://legalinstruments.oecd.org/en/instruments/OECD-LEGAL-0397. Accessed 5 Mar 2024."
},
{
"key": "9300_CR49",
"unstructured": "World Health Organization. Guidance for Research Ethics Committees for Rapid Review of Research During Public Health Emergencies. 2020. Available from: https://www.who.int/publications/i/item/9789240006218. Accessed 5 Mar 2024."
},
{
"DOI": "10.1080/14656566.2021.1898589",
"author": "WHK Schilling",
"doi-asserted-by": "crossref",
"first-page": "1257",
"issue": "10",
"journal-title": "Expert Opin Pharmacother",
"key": "9300_CR50",
"unstructured": "Schilling WHK, White NJ. Does hydroxychloroquine still have any role in the COVID-19 pandemic? Expert Opin Pharmacother. 2021;22(10):1257–66.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1111/j.1365-3156.2006.01765.x",
"author": "NJ White",
"doi-asserted-by": "crossref",
"first-page": "1483",
"issue": "10",
"journal-title": "Trop Med Int Health",
"key": "9300_CR51",
"unstructured": "White NJ. Clinical trials in tropical diseases: a politically incorrect view. Trop Med Int Health. 2006;11(10):1483–4.",
"volume": "11",
"year": "2006"
},
{
"DOI": "10.1038/s41541-020-00249-5",
"doi-asserted-by": "publisher",
"key": "9300_CR52",
"unstructured": "Simpson S, Chakrabarti A, Robinson D, Chirgwin K, Lumpkin M. Navigating facilitated regulatory pathways during a disease X pandemic. NPJ Vaccines. 2020;5(1):101. https://doi.org/10.1038/s41541-020-00249-5."
},
{
"DOI": "10.1136/bmj.m1575",
"doi-asserted-by": "publisher",
"key": "9300_CR53",
"unstructured": "Mak TK, Lim JC, Thanaphollert P, Mahlangu GN, Cooke E, Lumpkin MM. Global regulatory agility during COVID-19 and other health emergencies. BMJ. 2020;369(m1575):https://doi.org/10.1136/bmj.m1575."
},
{
"author": "R Harris",
"key": "9300_CR54",
"unstructured": "Harris R, Charles River Associates. Strengthening regulatory systems in LMICs: improving the sustainability of the vaccine innovation ecosystem in Africa. London: Wellcome Trust; 2022.",
"volume-title": "Strengthening regulatory systems in LMICs: improving the sustainability of the vaccine innovation ecosystem in Africa",
"year": "2022"
},
{
"DOI": "10.1111/bcp.14871",
"author": "S Tusino",
"doi-asserted-by": "crossref",
"first-page": "40",
"issue": "1",
"journal-title": "Br J Clin Pharmacol",
"key": "9300_CR55",
"unstructured": "Tusino S, Furfaro M. Rethinking the role of research ethics committees in the light of regulation (EU) no 536/2014 on clinical trials and the COVID‐19 pandemic. Br J Clin Pharmacol. 2022;88(1):40–6.",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1177/20563051211024977",
"doi-asserted-by": "publisher",
"key": "9300_CR56",
"unstructured": "Blevins JL, Edgerton E, Jason D, Lee JJ. Shouting into the wind: medical science versus “BS” in the Twitter Maelstrom of politics and misinformation about hydroxychloroquine. Social Media & Society. 2021;7(2):https://doi.org/10.1177/20563051211024977."
},
{
"DOI": "10.1016/S1473-3099(20)30866-5",
"doi-asserted-by": "publisher",
"key": "9300_CR57",
"unstructured": "Peiffer-Smadja N, Rebeaud ME, Guihur A, Mahamat-Saleh Y, Fiolet T. Hydroxychloroquine and COVID-19: a tale of populism and obscurantism. The Lancet Infectious Diseases. 2021;21(5):https://doi.org/10.1016/S1473-3099(20)30866-5."
},
{
"key": "9300_CR58",
"unstructured": "Minutes of the COPCOV DSMC, Compiled by Tim Peto. 23–24 May 2021."
},
{
"key": "9300_CR59",
"unstructured": "COPCOV 11am Meeting Minutes. 29 April 2020."
},
{
"key": "9300_CR60",
"unstructured": "COPCOV 11am Meeting Minutes. 12 June 2020."
},
{
"DOI": "10.1016/j.ijid.2020.05.093",
"author": "AU Shah",
"doi-asserted-by": "crossref",
"first-page": "108",
"journal-title": "Int J Infect Dis",
"key": "9300_CR61",
"unstructured": "Shah AU, Safri SN, Thevadas R, Noordin NK, Abd Rahman A, Sekawi Z, et al. COVID-19 outbreak in Malaysia: actions taken by the Malaysian government. Int J Infect Dis. 2020;97:108–16.",
"volume": "97",
"year": "2020"
},
{
"key": "9300_CR62",
"unstructured": "Gershkovich E, Sauer P. As Russian officials back hydroxychloroquine, doctors take matters into their own hands. The Moscow Times. 2020. Available from: https://www.themoscowtimes.com/2020/06/01/as-russian-officials-back-hydroxychloroquine-doctors-take-matters-into-their-own-hands-a70435. Accessed 5 Mar 2024."
},
{
"key": "9300_CR63",
"unstructured": "TASS. Russia removes hydroxychloroquine from list of drugs used to treat COVID-19. Russia News Agency. 7 May 2021. Available from: https://tass.com/society/1287237"
},
{
"DOI": "10.1016/S0140-6736(02)11477-2",
"author": "J Abraham",
"doi-asserted-by": "crossref",
"first-page": "1498",
"issue": "9344",
"journal-title": "Lancet",
"key": "9300_CR64",
"unstructured": "Abraham J. The pharmaceutical industry as a political player. Lancet. 2002;360(9344):1498–502.",
"volume": "360",
"year": "2002"
},
{
"key": "9300_CR65",
"unstructured": "MHRA Customer Services to Janelle Winters. FOI 22/944 – Freedom of information request – COPCOV. 29 September 2022"
},
{
"key": "9300_CR66",
"unstructured": "MHRA Customer Services to Janelle Winters. FOI 22/869 – Freedom of information request – COPCOV. 2022. Available from: https://www.gov.uk/government/publications/freedom-of-information-responses-from-the-mhra-week-commencing-29-august-2022/freedom-of-information-request-foi-22869. Accessed 19 Nov 2025."
},
{
"key": "9300_CR67",
"unstructured": "Matangila J. ANTICOV: initiating a platform adaptive trial for COVID outpatients in Africa. J Pub Health Africa. 2022;13(S1):52. Available from: https://publichealthinafrica.org/index.php/jphia/article/view/857/726. Accessed 1 June 2022."
},
{
"key": "9300_CR68",
"unstructured": "Organisation for Economic Co-operation and Development. Greater harmonization of clinical trial regulations would help the fight against COVID-19. 4 August 2010. Available from: https://www.oecd.org/coronavirus/policy-responses/greater-harmonisation-of-clinical-trial-regulations-would-help-the-fight-against-covid-19-732e1c5c/. Accessed 6 March 2024."
},
{
"DOI": "10.1163/9789004471641_008",
"doi-asserted-by": "crossref",
"key": "9300_CR69",
"unstructured": "Wamai RG, Shirley HC. The future of health in sub-Saharan Africa: is there a path to longer and healthier lives for all? In: Greiner C, van Wolputte S, Bollig M, editors. African futures. Leiden: Brill; 2022. p. 67–98."
},
{
"key": "9300_CR70",
"unstructured": "World Health Organization. First WHO Global Clinical Trials Forum puts forward a global vision for sustainable clinical research infrastructure. 29 November 2023. Available from: https://www.who.int/news/item/29-11-2023-first-who-global-clinical-trials-forum-puts-forward-a-global-vision-for-sustainable-clinical-research-infrastructure. Accessed 30 June 2023."
},
{
"key": "9300_CR71",
"unstructured": "Good Clinical Trials Collaborative. Guidance for good randomized clinical trials. May 2022. Available from: https://www.goodtrials.org/the-guidance/guidance-overview/. Accessed 6 March 2024."
},
{
"key": "9300_CR72",
"unstructured": "International Pandemic Preparedness Secretariat. 100 Days Mission: Implementation Report - 2022. London: Wellcome Trust; 2023. Available from: https://ippsecretariat.org/publication/second-implementation-report/. Accessed 10 Aug 2024."
},
{
"key": "9300_CR73",
"unstructured": "United Kingdom Department of Health & Social Care. Policy paper: Fit for the future: 10 year health plan for England – executive summary. 15 July 2025. Available from: https://www.gov.uk/government/publications/10-year-health-plan-for-england-fit-for-the-future/fit-for-the-future-10-year-health-plan-for-england-executive-summary. Accessed 18 July 2025."
},
{
"key": "9300_CR74",
"unstructured": "European Medicines Agency. Paving the way towards coordinated clinical trials in public health emergencies in the EU. 25 July 2023. Available from: https://www.ema.europa.eu/en/news/paving-way-towards-coordinated-clinical-trials-public-health-emergencies-eu#:~:text=EMA%20has%20published%20a%20report,EU)%20during%20public%20health%20emergencies. Accessed 1 July 2023."
},
{
"key": "9300_CR75",
"unstructured": "International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline Good Clinical Practice (GCP) E6(R3): Draft Version, Endorsed on 19 May 2023, Currently under Public Consultation. 2023. Available from: https://database.ich.org/sites/default/files/ICH_E6%28R3%29_DraftGuideline_2023_0519.pdf. Accessed 10 Aug 2024."
},
{
"DOI": "10.1016/S2214-109X(24)00515-1",
"author": "M Cavaleri",
"doi-asserted-by": "crossref",
"first-page": "e769",
"issue": "4",
"journal-title": "Lancet Glob Health",
"key": "9300_CR76",
"unstructured": "Cavaleri M, de Sousa CM, Hacker A, Higgs ES, Lumpkin MM, Maia CS, et al. A roadmap for fostering timely regulatory and ethics approvals of international clinical trials in support of global health research systems. Lancet Glob Health. 2025;13(4):e769–77.",
"volume": "13",
"year": "2025"
},
{
"key": "9300_CR77",
"unstructured": "Amuasi J. Research readiness for response as a foundation for effective research in global public health emergencies (panel presentation). Twelfth EDCTP Forum, Kigali, Rwanda. 16 June 2025."
},
{
"key": "9300_CR78",
"unstructured": "Harris R, Charles River Associates. Strengthening regulatory systems in LMICs: improving the sustainability of the vaccine innovation ecosystem in Africa. London: Wellcome Trust; 2022. Available from: https://cms.wellcome.org/sites/default/files/2022-07/strengthening-regulatory-systems-in-low-and-middle-income-countries-improving-the-sustainability-of-the-vaccine-innovation-ecosystem-in-africa.pdf. Accessed 18 July 2025."
},
{
"key": "9300_CR79",
"unstructured": "World Health Organization (WHO). WHO Global Benchmarking Tool (GBT) for Evaluation of National Regulatory System of Medical Products – Revision VI. 2021. Available from: https://www.who.int/publications/i/item/9789240020245. Accessed 10 Aug 2024."
},
{
"key": "9300_CR80",
"unstructured": "WHO. WHO Tool for Benchmarking Ethics Oversight of Health-Related Research with Human Participants, Draft Version for Piloting. 2022. Available from: https://cdn.who.int/media/docs/default-source/documents/ethics/rec-benchmarking-tool-for-piloting_21-september-2022.pdf. Accessed 10 Aug 2024."
},
{
"key": "9300_CR81",
"unstructured": "PANdemic preparedness platform for Health and Emerging infections Response (PANTHER). About PANTHER. Available from: https://pantherhealth.org/about/#why-panther. Accessed 18 July 2025."
},
{
"author": "A Petryna",
"key": "9300_CR82",
"unstructured": "Petryna A. When experiments travel: clinical trials and the global search for human subjects. Princeton: Princeton University Press; 2009.",
"volume-title": "When experiments travel: clinical trials and the global search for human subjects",
"year": "2009"
},
{
"DOI": "10.1111/dom.13088",
"author": "K Khunti",
"doi-asserted-by": "crossref",
"first-page": "427",
"issue": "2",
"journal-title": "Diabetes Obes Metab",
"key": "9300_CR83",
"unstructured": "Khunti K, Gomes MB, Pocock S, Shestakova MV, Pintat S, Fenici P, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–37.",
"volume": "20",
"year": "2018"
},
{
"DOI": "10.1111/j.1365-2796.2011.02351_2.x",
"author": "SN Whitney",
"doi-asserted-by": "crossref",
"first-page": "396",
"issue": "4",
"journal-title": "J Intern Med",
"key": "9300_CR84",
"unstructured": "Whitney SN, Schneider CE. A method to estimate the cost in lives of ethics board review of biomedical research. J Intern Med. 2011;269(4):396–402.",
"volume": "269",
"year": "2011"
},
{
"DOI": "10.1126/science.abc1731",
"author": "AJ London",
"doi-asserted-by": "crossref",
"first-page": "476",
"issue": "6490",
"journal-title": "Science",
"key": "9300_CR85",
"unstructured": "London AJ, Kimmelman J. Against pandemic research exceptionalism. Science. 2020;368(6490):476–7.",
"volume": "368",
"year": "2020"
}
],
"reference-count": 85,
"references-count": 85,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1186/s13063-025-09300-z"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Approval delays in multi-country COVID-19 trials: the case of COPCOV and the risk of therapeutic inertia",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy"
}
