A randomized trial of at-home COVID-19 tests, telemedicine, and rapid prescription delivery for immunocompromised individuals
et al., Research Square, doi:10.21203/rs.3.rs-5314583/v1, NCT05655546, Oct 2024
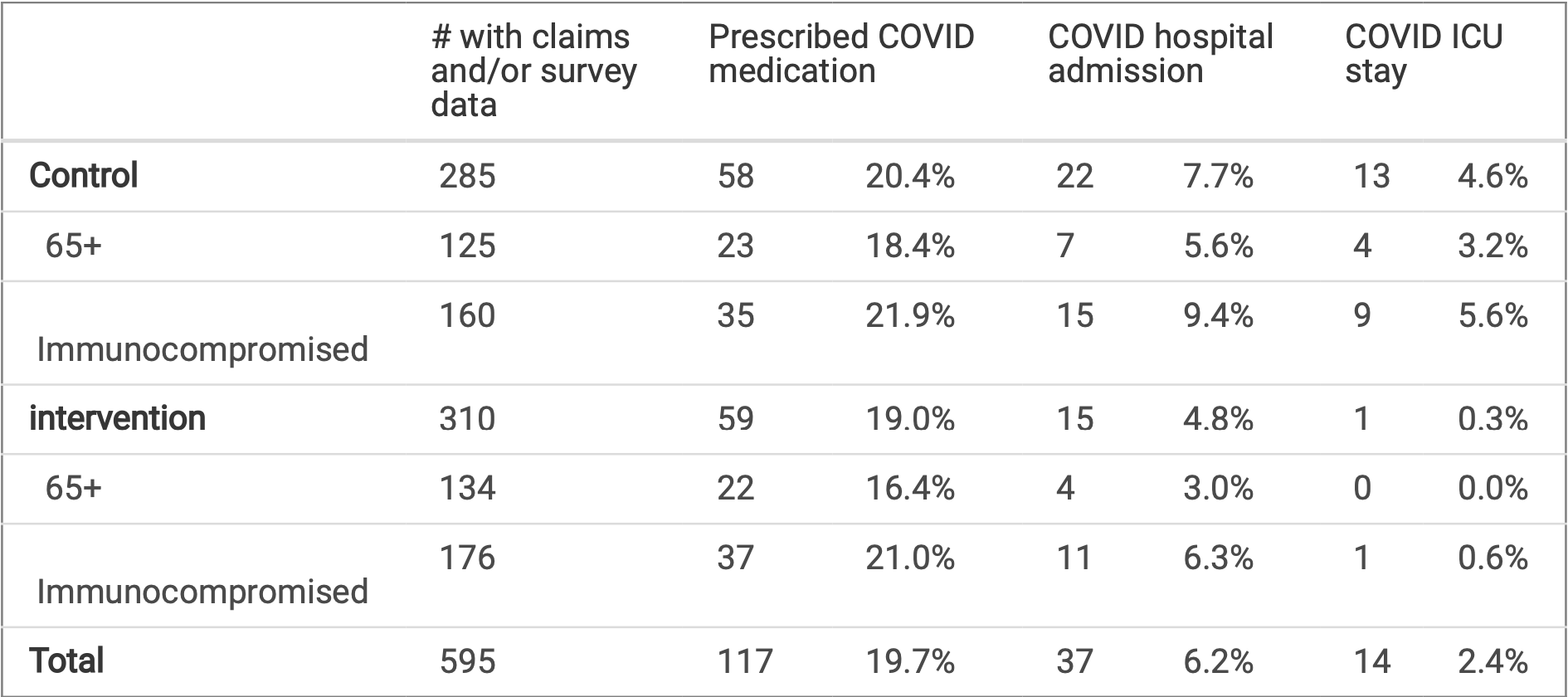
RCT 671 immunocompromised or aged 65+ participants in the US showing significantly reduced ICU admissions and cost of COVID care with access to at-home COVID tests, telemedicine, and rapid paxlovid delivery. There was no significant difference in COVID case rates or overall hospitalizations. The study ended early due to the intervention becoming unavailable, and had lower than intended sample size.
Vogel et al., 28 Oct 2024, Randomized Controlled Trial, USA, preprint, 7 authors, study period 1 December, 2022 - 16 May, 2024, trial NCT05655546 (history).
A randomized trial of at-home COVID-19 tests, telemedicine, and rapid prescription delivery for immunocompromised individuals
doi:10.21203/rs.3.rs-5314583/v1
Background: COVID-19 continues to impose substantial risks to people who are immunocompromised and over 65 years old. Objective: Using a randomized control trial, we evaluated whether access to at-home COVID-19 tests, telemedicine, and same-day prescription delivery could reduce COVID cases, hospitalizations, and the cost of COVID care for the high-risk populations. Design: Individuals participated remotely, half (n = 346) receiving the option to access 10 at-home COVID-19 tests per month for themselves and others in their household as well as telemedicine and same-day Paxlovid delivery, and half following their standard testing and treatment practices (n = 325). Data sources: Outcome data were collected from surveys, electronic health records (EHR) and claims. Results: Intensive care unit (ICU) admissions were signi cantly reduced for intervention participants vs. control participants, (0.3% vs 4.6%, p < 0.001). COVID case incidence did not signi cantly differ (19.0% vs 20.4%, p = 0.69), nor did hospitalizations (5.2% vs 7.7%, p = 0.14). The intervention was estimated to result in a reduction of $3,650 in the cost of COVID care per person. Limitations: The speci c intervention used is no longer available in the market and alternatives should be considered. Evolution of SARS-CoV-2 could change the effect observed. Survey completion is higher in the intervention group. Conclusions: In immunocompromised individuals and those at least aged 65 years, access to at-home COVID tests, telemedicine, and rapid Paxlovid delivery reduced the severity of COVID-19 infections, as re ected by a reduced need for ICU care; this has the potential to reduce the cost of COVID care.
requiring insurance preauthorization, may also improve outcomes. Based on these results and other data demonstrating the ongoing risk of COVID-19 to high-risk individuals [2, 21] , we recommend that payers and public health organizations provide COVID tests and rapid treatment to high-risk individuals, at no cost to the individuals, for as long as the virus continues to circulate.
Declarations
Data sharing Anonymized data can be shared upon request.
Competing Interests VK is an employee and shareholder of CareEvolution.
Author Contribution JMV, GQ, and ET designed the study in collaboration with Cue Health. JMV, EC, FD, and VK implemented the study design. JMV, TH, and GQ completed data analysis. JMV created the initial manuscript draft. All authors edited it and reviewed the nal version.
Control
Supplementary Files This is a list of supplementary les associated with this preprint. Click to download. suppCueOct22.docx
References
Barnes, Goodyear, Willicombe, Gaskell, Siebert et al., SARS-CoV-2-speci c immune responses and clinical outcomes after COVID-19 vaccination in patients with immunesuppressive disease, Nat Med
Cdc, Infection control guidance: SARS-CoV-2, COVID
Chi, Chiu, Chen, Weng, Lin, To PCR or not? The impact of shifting policy from PCR to rapid antigen tests to diagnose COVID-19 during the omicron epidemic: a nationwide surveillance study, Front Public Health
Chu, Schwartz, Donnelly, Chuey, Soto et al., Comparison of Home Antigen Testing With RT-PCR and Viral Culture During the Course of SARS-CoV-2 Infection, JAMA Intern Med
Corey, Beyrer, Cohen, Michael, Bedford et al., SARS-CoV-2 variants in patients with immunosuppression, N Engl J Med
Haidar, Mellors, Improving the Outcomes of Immunocompromised Patients With Coronavirus Disease 2019, Clin Infect Dis
Harpaz, Dahl, Dooling, Prevalence of Immunosuppression Among US Adults, 2013, JAMA
Hogan, Duerr, Dimartino, Marier, Hochman et al., Remdesivir Resistance in Transplant Recipients With Persistent Coronavirus Disease 2019, Clin Infect Dis
Ketkar, Willey, Glasser, Dobie, Wenziger et al., Assessing the burden and cost of COVID-19 across variants in commercially insured immunocompromised populations in the United States: Updated results and trends from the ongoing EPOCH-US study, Adv Ther
Lee, Wong, Chai, Lee, Lee et al., E cacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis, BMJ
Martinson, Lapham, Prevalence of Immunosuppression Among US Adults, JAMA
Nejad, Shobeiri, Dehghanbanadaki, Tabary, Aryannejad et al., Seroconversion following the rst, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: a systematic review and meta-analysis, Virol J
Pearce, Lim, Bythell, Lanyon, Hogg et al., Antibody prevalence after three or more COVID-19 vaccine doses in individuals who are immunosuppressed in the UK: a crosssectional study from MELODY, Lancet Rheumatol
Quer, Coughlin, Villacian, Delgado, Harris et al., Feasibility of wearable sensor signals and self-reported symptoms to prompt at-home testing for acute respiratory viruses in the USA (DETECT-AHEAD): a decentralised, randomised controlled trial, Lancet Digit Health
Quer, Radin, Gadaleta, Baca-Motes, Ariniello et al., Wearable sensor data and self-reported symptoms for COVID-19 detection, Nat Med
Robert, Vaca, Paredes Roger, Jorge, Brandon et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Romero Starke, Reissig, Petereit-Haack, Schmauder, Nienhaus et al., The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis, BMJ Glob Health, doi:10.1136/bmjgh-2021-006434
Se, Mellis, Grijalva, Talbot, Schmitz et al., SARS-CoV-2 viral shedding and rapid antigen test performance -respiratory virus transmission network, November 2022-may 2023, MMWR Morb Mortal Wkly Rep
Singson, Kirley, Pham, Rothrock, Armistead et al., Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19 -COVID-NET, 10 states, march 2020-February 2022, MMWR Morb Mortal Wkly Rep
Turtle, Thorpe, Drake, Swets, Palmieri et al., Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study, PLoS Med
Wallace, Kenney, Malani, Clauw, Nallamothu et al., Prevalence of immunosuppressive drug use among commercially insured US adults, 2018-2019, JAMA Netw Open
DOI record:
{
"DOI": "10.21203/rs.3.rs-5314583/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-5314583/v1",
"abstract": "<title>Abstract</title>\n <p><bold>Background: </bold>COVID-19 continues to impose substantial risks to people who are immunocompromised and over 65 years old.\n<bold>Objective: </bold>Using a randomized control trial, we evaluated whether access to at-home COVID-19 tests, telemedicine, and same-day prescription delivery could reduce COVID cases, hospitalizations, and the cost of COVID care for the high-risk populations.\n<bold>Design: </bold>Individuals participated remotely, half (n = 346) receiving the option to access 10 at-home COVID-19 tests per month for themselves and others in their household as well as telemedicine and same-day Paxlovid delivery, and half following their standard testing and treatment practices (n = 325).\n<bold>Data sources: </bold>Outcome data were collected from surveys, electronic health records (EHR) and claims.\n<bold>Results: </bold>Intensive care unit (ICU) admissions were significantly reduced for intervention participants vs. control participants, (0.3% vs 4.6%, p < 0.001). COVID case incidence did not significantly differ (19.0% vs 20.4%, p = 0.69), nor did hospitalizations (5.2% vs 7.7%, p = 0.14). The intervention was estimated to result in a reduction of $3,650 in the cost of COVID care per person.\n<bold>Limitations: </bold>The specific intervention used is no longer available in the market and alternatives should be considered. Evolution of SARS-CoV-2 could change the effect observed. Survey completion is higher in the intervention group.\n<bold>Conclusions: </bold>In immunocompromised individuals and those at least aged 65 years, access to at-home COVID tests, telemedicine, and rapid Paxlovid delivery reduced the severity of COVID-19 infections, as reflected by a reduced need for ICU care; this has the potential to reduce the cost of COVID care.</p>",
"accepted": {
"date-parts": [
[
2024,
10,
22
]
]
},
"author": [
{
"affiliation": [
{
"name": "Scripps Research Institute"
}
],
"family": "Vogel",
"given": "Julia Moore",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Scripps Research Institute"
}
],
"family": "Hung",
"given": "Ting-Yang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Scripps Research Institute"
}
],
"family": "Coughlin",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Scripps Research Institute"
}
],
"family": "Delgado",
"given": "Felipe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "CareEvolution (United States)"
}
],
"family": "Kheterpal",
"given": "Vik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Scripps Research Institute"
}
],
"family": "Quer",
"given": "Giorgio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Scripps Research Institute"
}
],
"family": "Topol",
"given": "Eric",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
10,
28
]
],
"date-time": "2024-10-28T07:04:29Z",
"timestamp": 1730099069000
},
"deposited": {
"date-parts": [
[
2024,
10,
28
]
],
"date-time": "2024-10-28T07:04:40Z",
"timestamp": 1730099080000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
10,
28
]
],
"date-time": "2024-10-28T08:10:34Z",
"timestamp": 1730103034518,
"version": "3.28.0"
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
10,
28
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
28
]
],
"date-time": "2024-10-28T00:00:00Z",
"timestamp": 1730073600000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-5314583/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-5314583/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"posted": {
"date-parts": [
[
2024,
10,
28
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2024,
10,
28
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1371/journal.pmed.1004086",
"article-title": "Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study",
"author": "Turtle L",
"doi-asserted-by": "crossref",
"first-page": "e1004086",
"journal-title": "PLoS Med",
"key": "ref1",
"unstructured": "Turtle L, Thorpe M, Drake TM, Swets M, Palmieri C, Russell CD, et al. Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023;20: e1004086.",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1007/s12325-023-02754-0",
"article-title": "Assessing the burden and cost of COVID-19 across variants in commercially insured immunocompromised populations in the United States: Updated results and trends from the ongoing EPOCH-US study",
"author": "Ketkar A",
"doi-asserted-by": "crossref",
"first-page": "1075",
"journal-title": "Adv Ther",
"key": "ref2",
"unstructured": "Ketkar A, Willey V, Glasser L, Dobie C, Wenziger C, Teng C-C, et al. Assessing the burden and cost of COVID-19 across variants in commercially insured immunocompromised populations in the United States: Updated results and trends from the ongoing EPOCH-US study. Adv Ther. 2024;41: 1075–1102.",
"volume": "41",
"year": "2024"
},
{
"DOI": "10.1136/bmjgh-2021-006434",
"article-title": "The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis",
"author": "Romero Starke K",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Glob Health",
"key": "ref3",
"unstructured": "Romero Starke K, Reissig D, Petereit-Haack G, Schmauder S, Nienhaus A, Seidler A. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Health. 2021;6. doi:10.1136/bmjgh-2021-006434",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1001/jama.2016.16477",
"article-title": "Prevalence of Immunosuppression Among US Adults, 2013",
"author": "Harpaz R",
"doi-asserted-by": "crossref",
"first-page": "2547",
"journal-title": "JAMA",
"key": "ref4",
"unstructured": "Harpaz R, Dahl RM, Dooling KL. Prevalence of Immunosuppression Among US Adults, 2013. JAMA. 2016;316: 2547–2548.",
"volume": "316",
"year": "2016"
},
{
"DOI": "10.1001/jama.2023.28019",
"article-title": "Prevalence of Immunosuppression Among US Adults",
"author": "Martinson ML",
"doi-asserted-by": "crossref",
"first-page": "880",
"journal-title": "JAMA",
"key": "ref5",
"unstructured": "Martinson ML, Lapham J. Prevalence of Immunosuppression Among US Adults. JAMA. 2024;331: 880–882.",
"volume": "331",
"year": "2024"
},
{
"key": "ref6",
"unstructured": "US Census Bureau. U.S. Older Population Grew From 2010 to 2020 at Fastest Rate Since 1880 to 1890. 2023 [cited 1 Aug 2024]. Available: https://www.census.gov/library/stories/2023/05/2020-census-united-states-older-population-grew.html"
},
{
"key": "ref7",
"unstructured": "CDC. Covid-net. In: COVID-19 [Internet]. 18 Jul 2024 [cited 1 Aug 2024]. Available: https://www.cdc.gov/covid/php/covid-net/index.html"
},
{
"article-title": "Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis",
"author": "Lee ARYB",
"first-page": "e068632",
"journal-title": "BMJ",
"key": "ref8",
"unstructured": "Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376: e068632.",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1016/S2665-9913(23)00160-1",
"article-title": "Antibody prevalence after three or more COVID-19 vaccine doses in individuals who are immunosuppressed in the UK: a cross-sectional study from MELODY",
"author": "Pearce FA",
"doi-asserted-by": "crossref",
"first-page": "e461",
"journal-title": "Lancet Rheumatol",
"key": "ref9",
"unstructured": "Pearce FA, Lim SH, Bythell M, Lanyon P, Hogg R, Taylor A, et al. Antibody prevalence after three or more COVID-19 vaccine doses in individuals who are immunosuppressed in the UK: a cross-sectional study from MELODY. Lancet Rheumatol. 2023;5: e461–e473.",
"volume": "5",
"year": "2023"
},
{
"DOI": "10.1186/s12985-022-01858-3",
"article-title": "Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: a systematic review and meta-analysis",
"author": "Mehrabi Nejad M-M",
"doi-asserted-by": "crossref",
"first-page": "132",
"journal-title": "Virol J",
"key": "ref10",
"unstructured": "Mehrabi Nejad M-M, Shobeiri P, Dehghanbanadaki H, Tabary M, Aryannejad A, Haji Ghadery A, et al. Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: a systematic review and meta-analysis. Virol J. 2022;19: 132.",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1038/s41591-023-02414-4",
"article-title": "SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease",
"author": "Barnes E",
"doi-asserted-by": "crossref",
"first-page": "1760",
"journal-title": "Nat Med",
"key": "ref11",
"unstructured": "Barnes E, Goodyear CS, Willicombe M, Gaskell C, Siebert S, I de Silva T, et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat Med. 2023;29: 1760–1774.",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciab397",
"article-title": "Improving the Outcomes of Immunocompromised Patients With Coronavirus Disease 2019",
"author": "Haidar G",
"doi-asserted-by": "crossref",
"first-page": "e1397",
"journal-title": "Clin Infect Dis",
"key": "ref12",
"unstructured": "Haidar G, Mellors JW. Improving the Outcomes of Immunocompromised Patients With Coronavirus Disease 2019. Clin Infect Dis. 2021;73: e1397–e1401.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1056/NEJMsb2104756",
"article-title": "SARS-CoV-2 variants in patients with immunosuppression",
"author": "Corey L",
"doi-asserted-by": "crossref",
"first-page": "562",
"journal-title": "N Engl J Med",
"key": "ref13",
"unstructured": "Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385: 562–566.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciac769",
"article-title": "Remdesivir Resistance in Transplant Recipients With Persistent Coronavirus Disease 2019",
"author": "Hogan JI",
"doi-asserted-by": "crossref",
"first-page": "342",
"journal-title": "Clin Infect Dis",
"key": "ref14",
"unstructured": "Hogan JI, Duerr R, Dimartino D, Marier C, Hochman SE, Mehta S, et al. Remdesivir Resistance in Transplant Recipients With Persistent Coronavirus Disease 2019. Clin Infect Dis. 2023;76: 342–345.",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients",
"author": "Gottlieb Robert L",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "ref15",
"unstructured": "Gottlieb Robert L., Vaca Carlos E., Paredes Roger, Mera Jorge, Webb Brandon J., Perez Gilberto, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2022;386: 305–315.",
"volume": "386",
"year": "2022"
},
{
"key": "ref16",
"unstructured": "CDC. Infection control guidance: SARS-CoV-2. In: COVID-19 [Internet]. 23 Jul 2024 [cited 1 Aug 2024]. Available: https://www.cdc.gov/covid/hcp/infection-control/index.html"
},
{
"key": "ref17",
"unstructured": "ImmunoCARE: Rapid, Accurate COVID Testing to Reduce Hospitalization of Immunocompromised Individuals. In: ClinicalTrials.Gov [Internet]. [cited 22 Jul 2024]. Available: https://clinicaltrials.gov/study/NCT05655546"
},
{
"DOI": "10.1001/jamanetworkopen.2021.4920",
"article-title": "Prevalence of immunosuppressive drug use among commercially insured US adults, 2018–2019",
"author": "Wallace BI",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "ref18",
"unstructured": "Wallace BI, Kenney B, Malani PN, Clauw DJ, Nallamothu BK, Waljee AK. Prevalence of immunosuppressive drug use among commercially insured US adults, 2018–2019. JAMA Netw Open. 2021;4: e214920.",
"volume": "4",
"year": "2021"
},
{
"key": "ref19",
"unstructured": "Center for Devices, Radiological Health. Do Not Use Cue Health’s COVID-19 Tests Due to Risk of False Results: FDA Safety Communication. In: U.S. Food and Drug Administration [Internet]. FDA; 22 Jul 2024 [cited 31 Jul 2024]. Available: https://www.fda.gov/medical-devices/safety-communications/do-not-use-cue-healths-covid-19-tests-due-risk-false-results-fda-safety-communication"
},
{
"key": "ref20",
"unstructured": "FH trackers: COVID-19 cost tracker. [cited 31 Jul 2024]. Available: https://www.fairhealth.org/fh-trackers/covid19-heatmap"
},
{
"DOI": "10.15585/mmwr.mm7127a3",
"article-title": "Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19 — COVID-NET, 10 states, march 2020–February 2022",
"author": "Singson JRC",
"doi-asserted-by": "crossref",
"first-page": "878",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "ref21",
"unstructured": "Singson JRC, Kirley PD, Pham H, Rothrock G, Armistead I, Meek J, et al. Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19 — COVID-NET, 10 states, march 2020–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71: 878–884.",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7316a2",
"article-title": "SARS-CoV-2 viral shedding and rapid antigen test performance — respiratory virus transmission network, November 2022–may 2023",
"author": "Smith-Jeffcoat SE",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "ref22",
"unstructured": "Smith-Jeffcoat SE, Mellis AM, Grijalva CG, Talbot HK, Schmitz J, Lutrick K, et al. SARS-CoV-2 viral shedding and rapid antigen test performance — respiratory virus transmission network, November 2022–may 2023. MMWR Morb Mortal Wkly Rep. 2024;73: 365–371.",
"volume": "73",
"year": "2024"
},
{
"DOI": "10.3389/fpubh.2023.1148637",
"article-title": "To PCR or not? The impact of shifting policy from PCR to rapid antigen tests to diagnose COVID-19 during the omicron epidemic: a nationwide surveillance study",
"author": "Chi H",
"doi-asserted-by": "crossref",
"first-page": "1148637",
"journal-title": "Front Public Health",
"key": "ref23",
"unstructured": "Chi H, Chiu N-C, Chen C-C, Weng S-L, Lien C-H, Lin C-H, et al. To PCR or not? The impact of shifting policy from PCR to rapid antigen tests to diagnose COVID-19 during the omicron epidemic: a nationwide surveillance study. Front Public Health. 2023;11: 1148637.",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1001/jamainternmed.2022.1827",
"article-title": "Comparison of Home Antigen Testing With RT-PCR and Viral Culture During the Course of SARS-CoV-2 Infection",
"author": "Chu VT",
"doi-asserted-by": "crossref",
"first-page": "701",
"journal-title": "JAMA Intern Med",
"key": "ref24",
"unstructured": "Chu VT, Schwartz NG, Donnelly MAP, Chuey MR, Soto R, Yousaf AR, et al. Comparison of Home Antigen Testing With RT-PCR and Viral Culture During the Course of SARS-CoV-2 Infection. JAMA Intern Med. 2022;182: 701–709.",
"volume": "182",
"year": "2022"
},
{
"key": "ref25",
"unstructured": "Assistant Secretary for Public Affairs (ASPA). Fact sheet: End of the COVID-19 public health emergency. In: US Department of Health and Human Services [Internet]. 9 May 2023 [cited 14 Aug 2024]. Available: https://www.hhs.gov/about/news/2023/05/09/fact-sheet-end-of-the-covid-19-public-health-emergency.html"
},
{
"DOI": "10.1038/s41591-020-1123-x",
"article-title": "Wearable sensor data and self-reported symptoms for COVID-19 detection",
"author": "Quer G",
"doi-asserted-by": "crossref",
"first-page": "73",
"journal-title": "Nat Med",
"key": "ref26",
"unstructured": "Quer G, Radin JM, Gadaleta M, Baca-Motes K, Ariniello L, Ramos E, et al. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat Med. 2021;27: 73–77.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/S2589-7500(24)00096-7",
"article-title": "Feasibility of wearable sensor signals and self-reported symptoms to prompt at-home testing for acute respiratory viruses in the USA (DETECT-AHEAD): a decentralised, randomised controlled trial",
"author": "Quer G",
"doi-asserted-by": "crossref",
"first-page": "e546",
"journal-title": "Lancet Digit Health",
"key": "ref27",
"unstructured": "Quer G, Coughlin E, Villacian J, Delgado F, Harris K, Verrant J, et al. Feasibility of wearable sensor signals and self-reported symptoms to prompt at-home testing for acute respiratory viruses in the USA (DETECT-AHEAD): a decentralised, randomised controlled trial. Lancet Digit Health. 2024;6: e546–e554.",
"volume": "6",
"year": "2024"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-5314583/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "A randomized trial of at-home COVID-19 tests, telemedicine, and rapid prescription delivery for immunocompromised individuals",
"type": "posted-content"
}