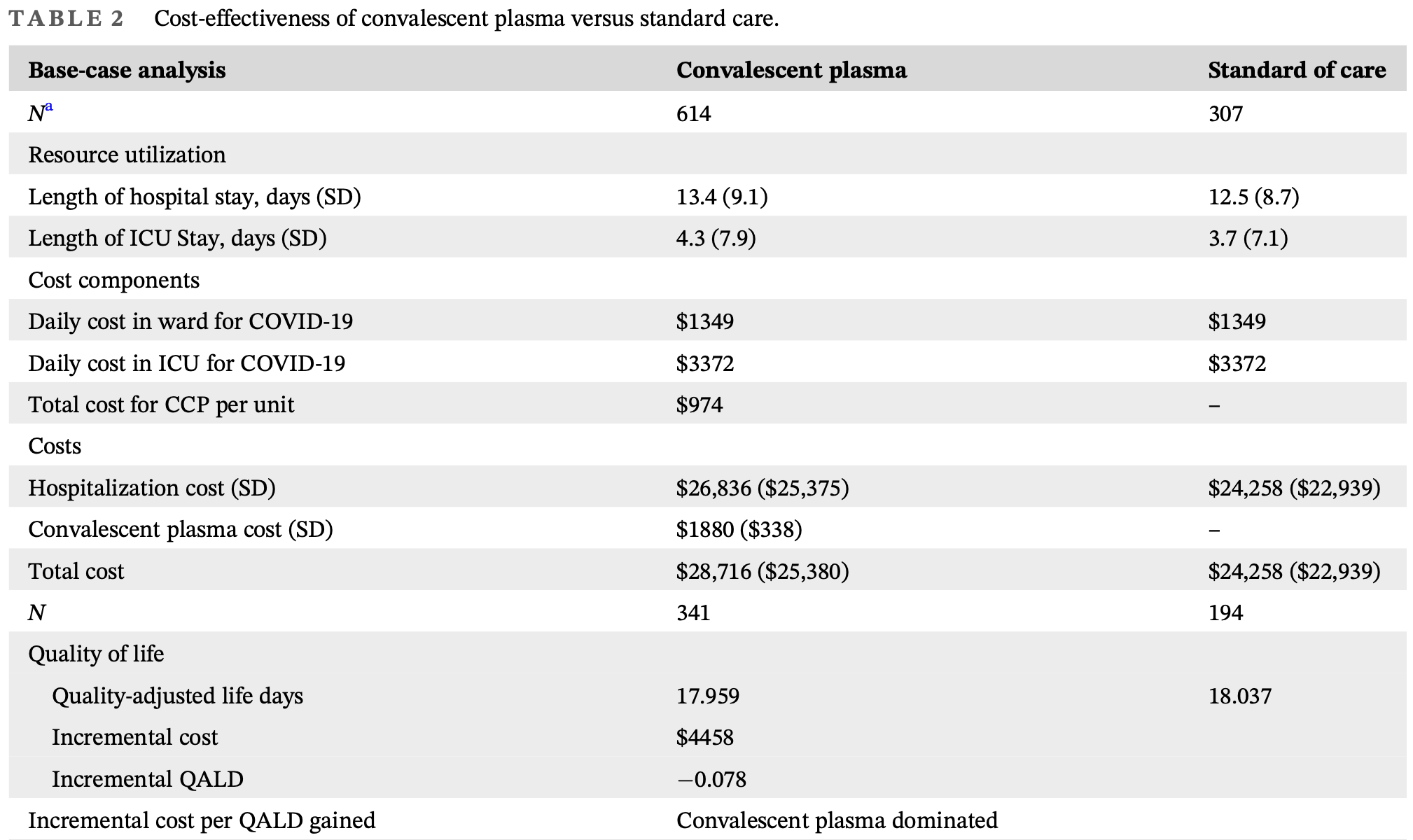
Quality of life and cost‐effectiveness of convalescent plasma compared to standard care for hospitalized COVID‐19 patients in the CONCOR‐1 trial
et al., Transfusion, doi:10.1111/trf.17777, NCT04348656, Mar 2024
Analysis of the CONCUR-1 RCT showing that convalescent plasma was associated with higher costs, longer hospital stays, and lower quality-adjusted life days.
Tse et al., 21 Mar 2024, Randomized Controlled Trial, Canada, peer-reviewed, 21 authors, study period 14 May, 2020 - 29 January, 2021, trial NCT04348656 (history).
Contact: fengxie@mcmaster.ca.
Quality of life and cost‐effectiveness of convalescent plasma compared to standard care for hospitalized COVID‐19 patients in the CONCOR‐1 trial
Transfusion, doi:10.1111/trf.17777
Background: The CONvalescent Plasma for Hospitalized Adults With COVID-19 Respiratory Illness (CONCOR-1) trial was a multicenter randomized controlled trial assessing convalescent plasma in hospitalized COVID-19 patients. This study evaluates the cost-effectiveness of convalescent plasma and its impact on quality-of-life to provide insight into its potential as an alternative treatment in resource-constrained settings. Methods: Individual patient data on health outcomes and resource utilization from the CONCOR-1 trial were used to conduct the analysis from the Canadian public payer's perspective with a time horizon of 30 days post-randomization. Baseline and 30-day EQ-5D-5L were measured to calculate quality-adjusted survival. All costs are presented in 2021 Canadian dollars. The base case assessed the EQ-5D-5L scores of hospitalized inpatients reporting at both timepoints, and a utility score of 0 was assigned for patients who died within 30 days. Costs for all patients enrolled were used. The sensitivity analysis utilizes EQ-5D-5L scores from the same population but only uses costs from this population. Results: 940 patients were randomized: 627 received CCP and 313 received standard care. The total costs were $28,716 (standard deviation, $25,380) and $24,258 ($22,939) for the convalescent plasma and standard care arms respectively. EQ-5D-5L scores were 0.61 in both arms (p = .85) at baseline. At 30 days, Abbreviations: AEs, adverse events; CCP, convalescent plasma; CONCOR-1, convalescent plasma for hospitalized adults with COVID-19 respiratory illness; ECMO,
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article.
References
Axfors, Janiaud, Schmitt, Van't Hooft, Smith et al., Association between convalescent plasma treatment and mortality in COVID-19: collaborative systematic review and meta-analysis of randomized clinical trials, BMC Infect Dis
Bégin, Callum, Heddle, Cook, Zeller et al., Convalescent plasma for adults with acute COVID-19 respiratory illness (CONCOR-1): study protocol for an international, multicentre, randomized, open-label trial, Trials
Bégin, Callum, Jamula, Cook, Heddle et al., Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med
De Oliveira, De Ávila, De Oliveira, Da, Severino Sampaio et al., Persistent symptoms, quality of life, and risk factors in long COVID: a crosssectional study of hospitalized patients in Brazil, Int J Infect Dis
Don, Michael, Federico, De B-G, Andrew et al., Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations, BMJ
Estcourt, Turgeon, Mcquilten, Mcverry, Al-Beidh et al., Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial, JAMA
Feng, Kohlmann, Janssen, Buchholz, Psychometric properties of the EQ-5D-5L: a systematic review of the literature, Qual Life Res
Fenwick, Claxton, Sculpher, Representing uncertainty: the role of cost-effectiveness acceptability curves, Health Econ
Hamilton, Lee, Arnold, Lilford, Hemming, Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial, Int J Infect Dis
Herdman, Gudex, Lloyd, Janssen, Kind et al., Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L), Qual Life Res
Information, COVID-19 hospitalization and emergency department statistics
Joyner, Carter, Senefeld, Klassen, Mills et al., Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med
Korley, Durkalski-Mauldin, Yeatts, Schulman, Davenport et al., Early convalescent plasma for high-risk outpatients with Covid-19, N Engl J Med
Lang-Meli, Fuchs, Mathé, Ho, Kern et al., Case series: convalescent plasma therapy for patients with COVID-19 and primary antibody deficiency, J Clin Immunol
Libster, Marc, Wappner, Coviello, Bianchi et al., Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med
Ortigoza, Yoon, Goldfeld, Troxel, Daily et al., Efficacy and safety of COVID-19 convalescent plasma in hospitalized patients: a randomized clinical trial, JAMA Intern Med
Piechotta, Chai, Valk, Doree, Monsef et al., Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database Syst Rev
Senefeld, Klassen, Ford, Senese, Wiggins et al., Use of convalescent plasma in COVID-19 patients with immunosuppression, Transfusion
Tsuzuki, Miyazato, Terada, Morioka, Ohmagari et al., Impact of long-COVID on health-related quality of life in Japanese COVID-19 patients, Health Qual Life Outcomes
Xie, Pullenayegum, Gaebel, Bansback, Bryan et al., A time trade-off-derived value set of the EQ-5D-5L for Canada, Med Care
DOI record:
{
"DOI": "10.1111/trf.17777",
"ISSN": [
"0041-1132",
"1537-2995"
],
"URL": "http://dx.doi.org/10.1111/trf.17777",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>The CONvalescent Plasma for Hospitalized Adults With COVID‐19 Respiratory Illness (CONCOR‐1) trial was a multicenter randomized controlled trial assessing convalescent plasma in hospitalized COVID‐19 patients. This study evaluates the cost‐effectiveness of convalescent plasma and its impact on quality‐of‐life to provide insight into its potential as an alternative treatment in resource‐constrained settings.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Individual patient data on health outcomes and resource utilization from the CONCOR‐1 trial were used to conduct the analysis from the Canadian public payer's perspective with a time horizon of 30 days post‐randomization. Baseline and 30‐day EQ‐5D‐5L were measured to calculate quality‐adjusted survival. All costs are presented in 2021 Canadian dollars. The base case assessed the EQ‐5D‐5L scores of hospitalized inpatients reporting at both timepoints, and a utility score of 0 was assigned for patients who died within 30 days. Costs for all patients enrolled were used. The sensitivity analysis utilizes EQ‐5D‐5L scores from the same population but only uses costs from this population.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>940 patients were randomized: 627 received CCP and 313 received standard care. The total costs were $28,716 (standard deviation, $25,380) and $24,258 ($22,939) for the convalescent plasma and standard care arms respectively. EQ‐5D‐5L scores were 0.61 in both arms (<jats:italic>p</jats:italic> = .85) at baseline. At 30 days, EQ‐5D‐5L scores were 0.63 and 0.64 for patients in the convalescent plasma and standard care arms, respectively (<jats:italic>p</jats:italic> = .46). The incremental cost was $4458 and the incremental quality‐adjusted life day was −0.078.</jats:p></jats:sec><jats:sec><jats:title>Discussion</jats:title><jats:p>Convalescent plasma was less effective and more costly than standard care in treating hospitalized COVID‐19.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/trf.17777"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-09-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-02-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-03-21"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Health Research Methods, Evidence, and Impact McMaster University Hamilton Ontario Canada"
}
],
"family": "Tse",
"given": "Preston",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Health Research Methods, Evidence, and Impact McMaster University Hamilton Ontario Canada"
}
],
"family": "Yan",
"given": "Jiajun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Michael G. DeGroote Centre for Transfusion Research McMaster University Hamilton Ontario Canada"
}
],
"family": "Liu",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Michael G. DeGroote Centre for Transfusion Research McMaster University Hamilton Ontario Canada"
}
],
"family": "Jamula",
"given": "Erin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9671-3501",
"affiliation": [
{
"name": "Michael G. DeGroote Centre for Transfusion Research McMaster University Hamilton Ontario Canada"
},
{
"name": "Department of Medicine McMaster University Hamilton Ontario Canada"
},
{
"name": "Canadian Blood Services Ottawa Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Heddle",
"given": "Nancy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9594-2532",
"affiliation": [
{
"name": "Medical Affairs and Innovation, Héma‐Québec Québec City Québec Canada"
}
],
"authenticated-orcid": false,
"family": "Bazin",
"given": "Renée",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Héma‐Québec Montreal Québec Canada"
},
{
"name": "Division of Hematology‐Oncology, Department of Pediatrics CHU Sainte‐Justine Montreal Québec Canada"
}
],
"family": "Robitaille",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Statistics and Actuarial Science University of Waterloo Waterloo Ontario Canada"
}
],
"family": "Cook",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Population Health and Optimal Health Practices Research Unit (Trauma‐Emergency‐Critical Care Medicine) CHU de Québec – Université Laval Research Center Québec City Québec Canada"
},
{
"name": "Division of Critical Care Medicine, Department of Anesthesiology and Critical Care Medicine Université Laval Québec City Québec Canada"
}
],
"family": "Turgeon",
"given": "Alexis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Canadian Blood Services Ottawa Ontario Canada"
},
{
"name": "Clinical Epidemiology Program Ottawa Hospital Research Institute Ottawa Ontario Canada"
},
{
"name": "Department of Medicine University of Ottawa Ottawa Ontario Canada"
}
],
"family": "Fergusson",
"given": "Dean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine Weill Cornell Medicine New York New York USA"
}
],
"family": "Glesby",
"given": "Marshall",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "UBC Health Team‐Based Care Vancouver British Columbia USA"
},
{
"name": "CIHR‐Strategy for Patient‐Oriented Research Ottawa Ontario Canada"
}
],
"family": "Loftsgard",
"given": "Kent Cadogan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8042-1494",
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine Weill Cornell Medicine New York New York USA"
}
],
"authenticated-orcid": false,
"family": "Cushing",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Centre Hospitalier de l'Université de Montréal Montreal Québec Canada"
},
{
"name": "Innovation Hub Centre de Recherche du Centre Hospitalier de l'Université de Montréal Montreal Québec Canada"
}
],
"family": "Chassé",
"given": "Michaël",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Sunnybrook Health Sciences Centre, Department of Medicine University of Toronto Toronto Ontario Canada"
}
],
"family": "Daneman",
"given": "Nick",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4992-5288",
"affiliation": [
{
"name": "Centre de Recherche du CHUM Montreal Québec Canada"
},
{
"name": "Département de Microbiologie, Infectiologie et Immunologie Université de Montréal Montreal Québec Canada"
}
],
"authenticated-orcid": false,
"family": "Finzi",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "New York Blood Center New York New York USA"
},
{
"name": "Weil Cornell Medical College New York New York USA"
}
],
"family": "Sachais",
"given": "Bruce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Centre Hospitalier de l'Université de Montréal Montreal Québec Canada"
},
{
"name": "Department of Pediatrics CHU Sainte‐Justine Montreal Québec Canada"
}
],
"family": "Bégin",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Canadian Blood Services Ottawa Ontario Canada"
},
{
"name": "Department of Pathology and Molecular Medicine Kingston Health Sciences Centre and Queen's University Kingston Ontario Canada"
},
{
"name": "Department of Laboratory Medicine and Molecular Diagnostics Sunnybrook Health Sciences Centre Toronto Ontario Canada"
},
{
"name": "Department of Laboratory Medicine and Pathobiology University of Toronto Toronto Ontario Canada"
}
],
"family": "Callum",
"given": "Jeannie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0943-8853",
"affiliation": [
{
"name": "Michael G. DeGroote Centre for Transfusion Research McMaster University Hamilton Ontario Canada"
},
{
"name": "Department of Medicine McMaster University Hamilton Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Arnold",
"given": "Donald M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3454-6266",
"affiliation": [
{
"name": "Department of Health Research Methods, Evidence, and Impact McMaster University Hamilton Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Xie",
"given": "Feng",
"sequence": "additional"
}
],
"container-title": "Transfusion",
"container-title-short": "Transfusion",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
21
]
],
"date-time": "2024-03-21T12:44:38Z",
"timestamp": 1711025078000
},
"deposited": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T19:00:49Z",
"timestamp": 1712689249000
},
"funder": [
{
"DOI": "10.13039/501100000024",
"award": [
"447352"
],
"doi-asserted-by": "publisher",
"name": "Canadian Institutes of Health Research"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
10
]
],
"date-time": "2024-04-10T00:36:52Z",
"timestamp": 1712709412506
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
3,
21
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2024,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
21
]
],
"date-time": "2024-03-21T00:00:00Z",
"timestamp": 1710979200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/trf.17777",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "606-614",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2024,
3,
21
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
21
]
]
},
"published-print": {
"date-parts": [
[
2024,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/NEJMoa2031893",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.1186/s13063-021-05235-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1001/jamainternmed.2021.6850",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.1186/s12879-021-06829-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1001/jama.2021.18178",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"article-title": "Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations",
"author": "Don H",
"journal-title": "BMJ",
"key": "e_1_2_9_9_1",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1007/s11136-011-9903-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1097/MLR.0000000000000447",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_11_1"
},
{
"key": "e_1_2_9_12_1",
"unstructured": "Information CIfH.COVID‐19 hospitalization and emergency department statistics [product release].2022."
},
{
"DOI": "10.1002/hec.635",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"DOI": "10.1056/NEJMoa2103784",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_14_1"
},
{
"article-title": "Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review",
"author": "Piechotta V",
"issue": "7",
"journal-title": "Cochrane Database Syst Rev",
"key": "e_1_2_9_15_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033700",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1016/j.ijid.2021.06.034",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.1007/s11136-020-02688-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"DOI": "10.1016/j.ijid.2022.07.063",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.1186/s12955-022-02033-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.1007/s10875-021-01193-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1111/trf.16525",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/trf.17777"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Hematology",
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Quality of life and <scp>cost‐effectiveness</scp> of convalescent plasma compared to standard care for hospitalized <scp>COVID</scp>‐19 patients in the <scp>CONCOR</scp>‐1 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "64"
}